Streptococcus pneumoniae: Invasion and Inflammation - PubMed (original) (raw)
Review
Streptococcus pneumoniae: Invasion and Inflammation
Allister J Loughran et al. Microbiol Spectr. 2019 Mar.
Abstract
Streptococcus pneumoniae (the pneumoccus) is the leading cause of otitis media, community-acquired pneumonia, and bacterial meningitis. The success of the pneumococcus stems from its ability to persist in the population as a commensal and avoid killing by immune system. This chapter first reviews the molecular mechanisms that allow the pneumococcus to colonize and spread from one anatomical site to the next. Then, it discusses the mechanisms of inflammation and cytotoxicity during emerging and classical pneumococcal infections.
Figures
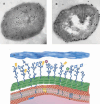
FIGURE 1
Immunohistochemical and schematic depiction of the choline biology of the pneumococcal surface. Immunogold labeling of pneumococci with (A) TEPC-15 antibody recognizing free choline and (B) antiautolysin antibody. These two images contrast free (A) versus CBP-bound (B) choline. (C) Schematic view of the capsule (blue), cell wall (green), and membrane (red). The teichoic and lipoteichoic acids are indicated as dark blue lines bearing choline (circles). A proportion of these are capped by choline-binding proteins. Courtesy of K.G. Murti, St. Jude Electron Microscopy Core Facility.
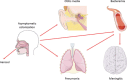
FIGURE 2
Schematic depiction of the spread and progression of S. pneumonia infection. Carriage of the pneumococcus occurs in the nasopharynx and is usually asymptomatic in healthy individuals. The bacteria are spread by aerosol from the nasopharynx of carriers. The pneumococcus can spread from the nasopharynx to a number of different tissues. In children the bacteria usually causes otitis media. Invasive diseases generally start in the lungs and spread to the blood, with the most serious complication being meningitis. The switch from asymptomatic colonization to invasive disease in healthy individuals usually occurs when there is a disruption in the innate immune defenses. (This figure contains some artwork produced by Servier Medical Art [
] under Creative Commons license 3.0).
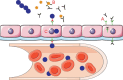
FIGURE 3
Schematic representation of the pneumococcus hijacking the pIgR/IgA system to cross the mucosal epithelia into the blood. (A) Mucosal epithelial cells transport IgA (black) from the basolateral to the apical surface using the receptor pIgR (green). This receptor is then endocytosed and recycled back to the basolateral surface to transport more IgA. (B) To protect itself from IgA, the pneumococcus produces the protease sIgA1 (yellow), which cleaves the host IgA into Fab fragments. (C) The choline-binding protein, CbpA (red), binds to the empty pIgR and shuttles the pneumococcus from the apical side to the basolateral side of the epithelial cells. (This figure contains some artwork produced by Servier Medical Art [
] under Creative Commons license 3.0).
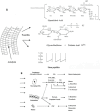
FIGURE 4
Structure of the pneumococcal cell wall and its relationship to inflammation. (A) Penicillin induces cell wall degradation by the autolysin releasing cell wall fragments such as lipoteichoic acid, glycan polymers with and without teichoic acid, and small stem peptides. All teichoicated species contain ChoP, a key component increasing inflammatory activity. (B) All of these components interact with a variety of human cells, which in turn produce inflammatory mediators. Particularly important in this response is the platelet activating factor (PAFr). These mediators combine to produce the symptomatology of pneumococcal infection, including changes in blood flow, fluid balance in the tissue, and leukocytosis. Glc, glucose; TDH, trideoxyhexose; NAcGaln, _N_-acetylgalctosamine; Galn, galactosamine;
l
-Ala,
l
-alanine;
d
-Glu,
d
-glucose;
l
-Lys,
l
-lysine; TNF, tumor necrosis factor; NO, nitric oxide; PGE2, prostaglandin E2; IC pressure, intracranial pressure; MIPS, macrophage inflammatory protein.
FIGURE 5
Domain structure of pneumolysin. Pneumolysin has three functionally separate domains: one activating complement, one causing hemolysis, and the other binding to cholesterol. Site-specific mutations alter these properties individually (191, 192).
Similar articles
- Upper Respiratory Tract Microbiome of Australian Aboriginal and Torres Strait Islander Children in Ear and Nose Health and Disease.
Coleman A, Zaugg J, Wood A, Cottrell K, Håkansson EG, Adams J, Brown M, Cervin A, Bialasiewicz S. Coleman A, et al. Microbiol Spectr. 2021 Oct 31;9(2):e0036721. doi: 10.1128/Spectrum.00367-21. Epub 2021 Oct 20. Microbiol Spectr. 2021. PMID: 34668729 Free PMC article. - Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - General health, otitis media, nasopharyngeal carriage and middle ear microbiology in Northern Territory Aboriginal children vaccinated during consecutive periods of 10-valent or 13-valent pneumococcal conjugate vaccines.
Leach AJ, Wigger C, Beissbarth J, Woltring D, Andrews R, Chatfield MD, Smith-Vaughan H, Morris PS. Leach AJ, et al. Int J Pediatr Otorhinolaryngol. 2016 Jul;86:224-32. doi: 10.1016/j.ijporl.2016.05.011. Epub 2016 May 11. Int J Pediatr Otorhinolaryngol. 2016. PMID: 27260611 - Interventions for the prevention of postoperative ear discharge after insertion of ventilation tubes (grommets) in children.
Syed MI, Suller S, Browning GG, Akeroyd MA. Syed MI, et al. Cochrane Database Syst Rev. 2013 Apr 30;2013(4):CD008512. doi: 10.1002/14651858.CD008512.pub2. Cochrane Database Syst Rev. 2013. PMID: 23633358 Free PMC article. Review. - Human airway macrophages are metabolically reprogrammed by IFN-γ resulting in glycolysis-dependent functional plasticity.
Cox DJ, Connolly SA, Ó Maoldomhnaigh C, Brugman AAI, Sandby Thomas O, Duffin E, Gogan KM, Ó Gallchobhair O, Murphy DM, O'Rourke SA, O'Connell F, Nadarajan P, Phelan JJ, Gleeson LE, Basdeo SA, Keane J. Cox DJ, et al. Elife. 2024 Dec 2;13:RP98449. doi: 10.7554/eLife.98449. Elife. 2024. PMID: 39620891 Free PMC article.
Cited by
- Daptomycin avoids drug resistance mediated by the BceAB transporter in Streptococcus pneumoniae.
Faure A, Manuse S, Gonin M, Grangeasse C, Jault J-M, Orelle C. Faure A, et al. Microbiol Spectr. 2024 Feb 6;12(2):e0363823. doi: 10.1128/spectrum.03638-23. Epub 2024 Jan 12. Microbiol Spectr. 2024. PMID: 38214521 Free PMC article. - In silico designing of a novel epitope-based candidate vaccine against Streptococcus pneumoniae with introduction of a new domain of PepO as adjuvant.
Bahadori Z, Shafaghi M, Madanchi H, Ranjbar MM, Shabani AA, Mousavi SF. Bahadori Z, et al. J Transl Med. 2022 Sep 4;20(1):389. doi: 10.1186/s12967-022-03590-6. J Transl Med. 2022. PMID: 36059030 Free PMC article. - The H9N2 avian influenza virus increases APEC adhesion to oviduct epithelia by viral NS1 protein-mediated activation of the TGF-β pathway.
Han J, Chang W, Fang J, Hou X, Li Z, Wang J, Deng W. Han J, et al. J Virol. 2024 Mar 19;98(3):e0151223. doi: 10.1128/jvi.01512-23. Epub 2024 Feb 28. J Virol. 2024. PMID: 38415626 Free PMC article. - SARS-CoV-2-Indigenous Microbiota Nexus: Does Gut Microbiota Contribute to Inflammation and Disease Severity in COVID-19?
Chattopadhyay I, Shankar EM. Chattopadhyay I, et al. Front Cell Infect Microbiol. 2021 Mar 11;11:590874. doi: 10.3389/fcimb.2021.590874. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33791231 Free PMC article. - Heat-Killed Fusobacterium nucleatum Triggers Varying Heme-Related Inflammatory and Stress Responses Depending on Primary Human Respiratory Epithelial Cell Type.
Koike R, Cueno ME, Nodomi K, Tamura M, Kamio N, Tanaka H, Kotani A, Imai K. Koike R, et al. Molecules. 2020 Aug 24;25(17):3839. doi: 10.3390/molecules25173839. Molecules. 2020. PMID: 32847022 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical