Randomized, Double-Blind, Placebo-Controlled Trial of Intraarticular Trans-Capsaicin for Pain Associated With Osteoarthritis of the Knee - PubMed (original) (raw)
Clinical Trial
. 2019 Sep;71(9):1524-1533.
doi: 10.1002/art.40894. Epub 2019 Jul 17.
Affiliations
- PMID: 30888737
- PMCID: PMC6772016
- DOI: 10.1002/art.40894
Clinical Trial
Randomized, Double-Blind, Placebo-Controlled Trial of Intraarticular Trans-Capsaicin for Pain Associated With Osteoarthritis of the Knee
Randall M Stevens et al. Arthritis Rheumatol. 2019 Sep.
Abstract
Objective: To assess the efficacy and safety of high-purity synthetic trans-capsaicin (CNTX-4975) in patients with chronic moderate-to-severe osteoarthritis (OA)-associated knee pain.
Methods: In this phase II multicenter double-blind study, patients ages 45-80 years who had stable knee OA were randomized in a 2:1:2 ratio to receive a single intraarticular injection of placebo, CNTX-4975 0.5 mg, or CNTX-4975 1.0 mg. The primary efficacy end point was area under the curve (AUC) for change from baseline in daily Western Ontario and McMaster Universities Osteoarthritis Index pain with walking score (range 0-10, 0 = none and 10 = extreme) through week 12. Secondary efficacy end points included a similar AUC analysis of outcomes in patients treated with CNTX-4975 0.5 mg, and evaluations extending to 24 weeks.
Results: Efficacy was evaluated in 172 patients (placebo group, n = 69; CNTX-4975 0.5 mg group, n = 33; CNTX-4975 1.0 mg group, n = 70). At week 12, greater decreases in the AUC for the pain score were observed with CNTX-4975 in the 0.5 mg and 1.0 mg groups versus placebo (0.5 mg group least squares mean difference [LSMD] -0.79, P = 0.0740; 1.0 mg group LSMD -1.6, P < 0.0001). Significant improvements were maintained at week 24 in the 1.0 mg group (LSMD -1.4, P = 0.0002). Treatment-emergent adverse events were similar in the placebo and CNTX-4975 1.0 mg groups.
Conclusion: In this study, CNTX-4975 provided dose-dependent improvement in knee OA-associated pain. CNTX-4975 1.0 mg produced a significant decrease in OA knee pain through 24 weeks; CNTX-4975 0.5 mg significantly improved pain at 12 weeks, but the effect was not evident at 24 weeks.
Trial registration: ClinicalTrials.gov NCT02558439.
© 2019 Centrexion Therapeutics Corporation. Arthritis & Rheumatology published by Wiley Periodicals, Inc. on behalf of American College of Rheumatology.
Figures
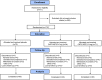
Figure 1
Disposition of the study patients. Reasons for exclusion at screening included Kellgren/Lawrence grade outside of range 2–4 (320 patients [60%]); inability to understand and follow study requirements, including diary entry via computer (64 [12%]); failure to meet the requirement for moderate‐to‐severe pain (29 [5%]); history of allergic reaction to the planned local anesthesia regimens, polyethylene glycol, or capsaicin (19 [3%]); baseline and screening scores outside of a 5–9 range on the Western Ontario and McMaster Universities Osteoarthritis Index (
WOMAC
) pain with walking assessment (12 [2%]); >2‐point difference in
WOMAC
pain with walking score between screening and baseline (11 [2%]); prior participation in an
ALGRX
4975 or
CNTX
‐4975 study (10 [2%]); and positive urine drug screen or active/past substance use disorder within prior year (10 [2%]). Other inclusion/exclusion criteria each contributed ≤1% to exclusions at screening. * = Number of patients in the safety analysis. † Three patients were excluded from the efficacy analysis (modified intent‐to‐treat population, n = 172). One patient was excluded (prior to unblinding) due to deviation/noncompliance, as this patient was injected at 2 different study sites (CNTX‐4975 1.0 mg, n = 1; placebo, n = 1). A third patient was lost to follow‐up in the CNTX‐4975 0.5 mg group.
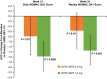
Figure 2
Western Ontario and McMaster Universities Osteoarthritis Index (
WOMAC
) pain with walking on a flat surface (
QA
- scores. Standardized area under the curve (
AUC
), normalized to the 0–10 rating scale, for change from baseline with
CNTX
‐4975 versus placebo in daily pain with walking scores through week 12 and in average weekly pain with walking scores through week 24 were evaluated. Analysis of covariance was performed in the modified intent‐to‐treat population.
LSMD
= least squares mean difference; 90%
CI
= 90% confidence interval.
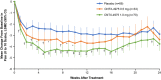
Figure 3
Change in average weekly
WOMAC
pain with walking scores. Change from baseline in average weekly scores through week 24 in patients treated with
CNTX
‐4975 versus placebo is shown. A mixed model for repeated measures was used in the modified intent‐to‐treat population. Week 12 was the prespecified landmark end point; other P values were considered nominal and are presented for summary purposes only. Baseline scores (range 0–10): placebo 7.4,
CNTX
‐4975 0.5 mg 7.2,
CNTX
‐4975 1.0 mg 7.2. * = P < 0.1; † = P < 0.05; ‡ = P < 0.001, versus placebo. See Figure 2 for definitions.
Comment in
- Trans-capsaicin for Pain Reduction and Improvement in Function in Patients With Knee Osteoarthritis: Comment on the Article by Stevens et al.
Yu Y, Lu ST. Yu Y, et al. Arthritis Rheumatol. 2020 Aug;72(8):1404-1405. doi: 10.1002/art.41286. Epub 2020 Jun 4. Arthritis Rheumatol. 2020. PMID: 32293119 No abstract available. - Reply.
Stevens RM, Campbell JN, Hanson PD. Stevens RM, et al. Arthritis Rheumatol. 2020 Aug;72(8):1405-1406. doi: 10.1002/art.41287. Epub 2020 May 31. Arthritis Rheumatol. 2020. PMID: 32301590 No abstract available.
Similar articles
- Single intra-articular injection of lightly cross-linked hyaluronic acid reduces knee pain in symptomatic knee osteoarthritis: a multicenter, double-blind, randomized, placebo-controlled trial.
Petterson SC, Plancher KD. Petterson SC, et al. Knee Surg Sports Traumatol Arthrosc. 2019 Jun;27(6):1992-2002. doi: 10.1007/s00167-018-5114-0. Epub 2018 Aug 29. Knee Surg Sports Traumatol Arthrosc. 2019. PMID: 30159738 Clinical Trial. - Efficacy and safety of single-dose onabotulinumtoxinA in the treatment of symptoms of osteoarthritis of the knee: results of a placebo-controlled, double-blind study.
McAlindon TE, Schmidt U, Bugarin D, Abrams S, Geib T, DeGryse RE, Kim K, Schnitzer TJ. McAlindon TE, et al. Osteoarthritis Cartilage. 2018 Oct;26(10):1291-1299. doi: 10.1016/j.joca.2018.05.001. Epub 2018 May 9. Osteoarthritis Cartilage. 2018. PMID: 29753118 Clinical Trial. - Towards a mechanism-based approach to pain management in osteoarthritis.
Malfait AM, Schnitzer TJ. Malfait AM, et al. Nat Rev Rheumatol. 2013 Nov;9(11):654-64. doi: 10.1038/nrrheum.2013.138. Epub 2013 Sep 17. Nat Rev Rheumatol. 2013. PMID: 24045707 Free PMC article. Review. - What is new in pain modification in osteoarthritis?
Miller RE, Block JA, Malfait AM. Miller RE, et al. Rheumatology (Oxford). 2018 May 1;57(suppl_4):iv99-iv107. doi: 10.1093/rheumatology/kex522. Rheumatology (Oxford). 2018. PMID: 29361112 Free PMC article. Review.
Cited by
- Therapeutic Advances in Diabetes, Autoimmune, and Neurological Diseases.
Liu J, Ting JP, Al-Azzam S, Ding Y, Afshar S. Liu J, et al. Int J Mol Sci. 2021 Mar 10;22(6):2805. doi: 10.3390/ijms22062805. Int J Mol Sci. 2021. PMID: 33802091 Free PMC article. Review. - Neural and immune roles in osteoarthritis pain: Mechanisms and intervention strategies.
Zou Y, Liu C, Wang Z, Li G, Xiao J. Zou Y, et al. J Orthop Translat. 2024 Aug 7;48:123-132. doi: 10.1016/j.jot.2024.07.010. eCollection 2024 Sep. J Orthop Translat. 2024. PMID: 39220678 Free PMC article. Review. - Mechanisms of Peripheral and Central Sensitization in Osteoarthritis Pain.
Ohashi Y, Uchida K, Fukushima K, Inoue G, Takaso M. Ohashi Y, et al. Cureus. 2023 Feb 22;15(2):e35331. doi: 10.7759/cureus.35331. eCollection 2023 Feb. Cureus. 2023. PMID: 36846635 Free PMC article. Review. - Osteoarthritis Pain.
Yu H, Huang T, Lu WW, Tong L, Chen D. Yu H, et al. Int J Mol Sci. 2022 Apr 22;23(9):4642. doi: 10.3390/ijms23094642. Int J Mol Sci. 2022. PMID: 35563035 Free PMC article. Review. - Peedanil Gold, Herbo-Mineral Formulation, Moderates Cytokine Levels and Attenuates Pathophysiology in Monosodium Iodoacetate Induced Osteoarthritis in SD Rat Model.
Balkrishna A, Sinha S, Karumuri S, Srivastava J, Haldar S, Varshney A. Balkrishna A, et al. Front Pharmacol. 2022 May 4;13:883475. doi: 10.3389/fphar.2022.883475. eCollection 2022. Front Pharmacol. 2022. PMID: 35600853 Free PMC article.
References
- McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma‐Zeinstra SM, et al. OARSI guidelines for the non‐surgical management of knee osteoarthritis. Osteoarthritis Cartilage 2014;22:363–88. - PubMed
- Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64:465–74. - PubMed
- Rutjes AW, Jüni P, da Costa BR, Trelle S, Nüesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta‐analysis. Ann Intern Med 2012;157:180–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical