Age-related inflammation triggers skeletal stem/progenitor cell dysfunction - PubMed (original) (raw)
. 2019 Apr 2;116(14):6995-7004.
doi: 10.1073/pnas.1810692116. Epub 2019 Mar 20.
Vivian Bradaschia-Correa 1, Sooyeon Lee 1, Kevin Leclerc 1, Karan S Patel 1, Emma Muinos Lopez 1, Hannah P Litwa 1, Shane S Neibart 1, Manasa Kadiyala 1, Madeleine Z Wong 1, Matthew M Mizrahi 1, Nury L Yim 1, Austin J Ramme 1, Kenneth A Egol 1, Philipp Leucht 3 2
Affiliations
- PMID: 30894483
- PMCID: PMC6452701
- DOI: 10.1073/pnas.1810692116
Age-related inflammation triggers skeletal stem/progenitor cell dysfunction
Anne Marie Josephson et al. Proc Natl Acad Sci U S A. 2019.
Abstract
Aging is associated with impaired tissue regeneration. Stem cell number and function have been identified as potential culprits. We first demonstrate a direct correlation between stem cell number and time to bone fracture union in a human patient cohort. We then devised an animal model recapitulating this age-associated decline in bone healing and identified increased cellular senescence caused by a systemic and local proinflammatory environment as the major contributor to the decline in skeletal stem/progenitor cell (SSPC) number and function. Decoupling age-associated systemic inflammation from chronological aging by using transgenic _Nfkb1_KO mice, we determined that the elevated inflammatory environment, and not chronological age, was responsible for the decrease in SSPC number and function. By using a pharmacological approach inhibiting NF-κB activation, we demonstrate a functional rejuvenation of aged SSPCs with decreased senescence, increased SSPC number, and increased osteogenic function. Unbiased, whole-genome RNA sequencing confirmed the reversal of the aging phenotype. Finally, in an ectopic model of bone healing, we demonstrate a functional restoration of regenerative potential in aged SSPCs. These data identify aging-associated inflammation as the cause of SSPC dysfunction and provide mechanistic insights into its reversal.
Keywords: bone healing; inflammation; regeneration; senescence; skeletal stem cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
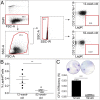
Fig. 1.
Skeletal stem/progenitor cell frequency declines in the aging patient. (A) FACS analysis of ICBG samples from 36 patients (20 male and 16 female) of varying ages revealed a significant (P < 0.05) negative correlation between age and SSPC number. (B) SSPC frequency is significantly decreased in patients older than 50 y of age (P < 0.05). (C) SSPC number is negatively correlated with time to bony union (P < 0.05). Green dots identify fractures that healed clinically and radiographically within 6 mo. Red dots mark patients with fracture union after 6 mo.
Fig. 2.
Aging results in impaired bone healing. (A and B) Histological sections of tibial monocortical defects 14 d after injury in 12-wk-old and 52-wk-old WT mice stained with Movat’s pentachrome. (C and D) Aniline blue staining depicting bone matrix deposition within the cortical defect. (E and F) Three-dimensional μCT reconstructions with surface rendering of bony regenerate (red) showing smaller callus size in the defect site of middle-aged animals. (G) Analysis of μCT data showing BV/TV (n = 6, P < 0.001), Tb.N (n = 6, P < 0.001), Tb.Th (n = 6, P < 0.05), and Tb.Sp (n = 6, P < 0.001) at postoperative day (POD) 7 and POD 14 in young and middle-aged mice. bm, bone marrow; c, cortical bone; is, injury site.
Fig. 3.
SSPC frequency declines with aging. (A) Representative FACS plots of young and middle-aged skeletal elements showing decrease in LepR+ SSPCs with aging. (B) Summary plot of FACS data demonstrating decrease in SSPC frequency in 52-wk-old (wo) mice (n = 5, P < 0.01). (C) cfu assay of young and middle-aged bone marrow showing representative colony staining and graphical depiction of quantification (n = 3, P < 0.0001).
Fig. 4.
Systemic cytokines are responsible for aging phenotype. (A) Schematic illustration depicting homochronic (young serum/young cells) and heterochronic (serum from middle-aged mice/young cells) in vitro culture conditions. (B) Cell proliferation assay of young SSPCs treated with sera from young or middle-aged mice showing an inhibitory effect of middle-aged sera on mitotic activity (n = 5). (C) Young SSPCs subjected to middle-aged serum exhibit more cell senescence at 4 d (n = 3, P < 0.01) and 7 d (n = 3, P < 0.001) as measured by SA-β-gal staining. (D) qRT-PCR shows induction of the expression of senescence-associated genes Il1a, Tnfa, and Rela in cells subjected to sera from middle-aged mice (n = 3, P < 0.05). (E_–_G) SA-β-gal staining of SSPCs from 12- and 52-wk-old mice showing increased senescence (arrowheads) in the aging animal. Quantification reveals a significant increase in senescence at 52 wk of age (n = 4, P < 0.01). (H) Consistent with the SA-β-gal staining, expression levels of Cdkn2a (p16) and cdkn1a (p21) were elevated in the middle-aged bone compartment (n = 7, P < 0.05). (I) Western blot for p-p65 reveals increased NF-κB activation in the middle-aged SSPCs (n = 3, P < 0.05). (J) Immunofluorescence of NF-κBp65 in young and middle-aged SSPCs revealed nuclear localization of NF-κBp65 in the middle-aged cells. (K) Quantification of NF-κBp65 (p65) nuclear localization demonstrates increased NF-κB activation in 52-wk-old SSPCs (n = 3, P < 0.001).
Fig. 5.
Young Nfkb1_−/_− mice mimic aging phenotype of SSPCs. (A) qRT-PCR of bone tissue from 30-wk-old Nfkb1_−/_− and age-matched WT mice revealing proinflammatory SASP-like phenotype in Nfkb1_−/_− mice (n = 3, P < 0.05). (B) Nfkb1_−/_− mice show characteristic decline of SSPC number at younger age (n = 4, P < 0.05). (C) Proliferation assay of WT and Nfkb1_−/_− SSPCs showing linear increase in cell number for WT cells and a steady state for Nfkb1_−/_− cells (n = 4). (D) Alizarin red staining of mineralization assay for WT and Nfkb1_−/_− cells in osteogenic media (OM) and GM. (E) Quantification of Alizarin red and alkaline phosphatase staining showing reduced osteogenic differentiation of Nfkb1_−/_− cells (n = 4, ***P < 0.001). (F) Expression analysis for osteogenic genes of WT and Nfkb1_−/_− cells treated with osteogenic differentiation media (n = 4, **P < 0.01 and ***P < 0.001).
Fig. 6.
Antiinflammatory treatment reverts inflamm-aging phenotype and increases SSPC pool. (A) Serum cytokine levels of IFN-γ (P < 0.05), TNF-α (P < 0.01), and IL-6 (P < 0.01) in young and middle-aged WT mice (n = 10). (B) Antiinflammatory drug treatment with salicylate reverses inflamm-aging phenotype (n = 6, P < 0.05). (C) Immunofluorescence for NF-κBp65 reveals nuclear localization of NF-κBp65 after treatment with middle-aged serum and a decrease in nuclear localization of NF-κBp65 in cells subjected to serum from middle-aged NSAID-treated mice. Quantification confirms increase in NF-κB activation with aging and decrease to juvenile levels after NSAID treatment (n = 4, P < 0.01). (D) Western blot for p-p65 confirming the decreased NF-κB activation in response to NSAID treatment (n = 3, *P < 0.05 and **P < 0.01). (E) SA-β-gal staining of young, middle-aged, and salicylate-treated middle-aged SSPCs show reversal of senescence phenotype in aging animals (n = 3, P < 0.05). (F) Graph showing SSPC frequency in young, middle-aged, and salicylate-treated middle-aged mice (n = 11, **P < 0.01 and ***P < 0.001). (G) Cfu-forming assay confirms increase of SSPC frequency after salicylate treatment (n = 3, P < 0.001). tx, treated; wo, weeks old.
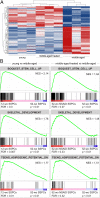
Fig. 7.
RNA-seq analysis reveals a shift toward increased stemness and osteogenesis and decreased adipogenesis in middle-aged antiinflammatory-treated mice. (A) Heat map representing gene-expression values of the top 133 genes with a false discovery rate-adjusted P value less than 0.01 (q > 0.01) across all samples. Hierarchical clustering of these genes reveals that LepR+ SSPCs isolated from 52-wk-old antiinflammatory-treated mice cluster with 12-wk-old LepR+ SSPCs. Columns indicate single samples, and rows indicate genes. (B) GSEA plots demonstrate that young SSPCs and middle-aged treated SSPCs positively correlate with gene sets for stemness (BOQUEST STEM CELL UP), osteogenesis (SKELETAL DEVELOPMENT), and decreased adipogenic potential (TSENG ADIPOGENIC POTENTIAL DN).
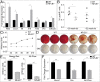
Fig. 8.
SSPC function is restored in aging animals after antiinflammatory treatment. (A) qRT-PCR of SSPCs shows increased osteogenic gene expression after repression of inflamm-aging (n = 3, *P < 0.05, **P < 0.01, and ***P < 0.001). (B) Representative images and quantification of osteogenic differentiation analyzed by Alizarin red staining (n = 3, P < 0.001). (C) Adipogenic differentiation analyzed by qRT-PCR for adipogenic markers (n = 3, P < 0.05) and (D) Oil Red O staining (representative images and quantification; n = 3, **P < 0.01 and ***P < 0.001). (E) Histomorphometry for adipocyte number in the tibial bone marrow of young, middle-aged, and middle-aged NSAID-treated mice (n = 5, *P < 0.05 and ***P < 0.001). (F and G) Pentachrome histology of renal capsule (rc) transplants of 52-wk-old (wo) untreated and salicylate-treated SSPCs into young WT host mice. (F) Histomorphometry shows a decrease in regenerate size in middle-aged mice and restoration of regenerative function after salicylate treatment (n = 4, P < 0.05). bg, bone graft; tx, treated.
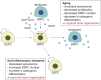
Fig. 9.
Schematic illustration of the cellular mechanism leading to impaired bone regeneration in elderly subjects. During aging, senescent SSPCs secrete SASP factors, which result in activation of NF-κB in adjacent SSPCs, inducing them to undergo senescence. This accumulation of senescent SSPCs leads to decreased SSPC self-renewal and proliferation, resulting in a decrease in overall number. Together with a decline in osteogenic differentiation, this leads to impaired bone regeneration in elderly subjects. NSAID treatment inhibits NF-κB activation, thereby blocking the effect of the SASP factors on surrounding SSPCs, which leads to a decrease in SSPC senescence, increase in SSPC number, improved osteogenic differentiation, and, finally, enhanced bone regeneration.
Comment in
- Bone inflamm-ageing.
Bernard NJ. Bernard NJ. Nat Rev Rheumatol. 2019 May;15(5):252. doi: 10.1038/s41584-019-0216-8. Nat Rev Rheumatol. 2019. PMID: 30948843 No abstract available.
Similar articles
- Age-related secretion of grancalcin by macrophages induces skeletal stem/progenitor cell senescence during fracture healing.
Zou NY, Liu R, Huang M, Jiao YR, Wei J, Jiang Y, He WZ, Huang M, Xu YL, Liu L, Sun YC, Yang M, Guo Q, Huang Y, Su T, Xiao Y, Wang WS, Zeng C, Lei GH, Luo XH, Li CJ. Zou NY, et al. Bone Res. 2024 Jan 25;12(1):6. doi: 10.1038/s41413-023-00309-1. Bone Res. 2024. PMID: 38267422 Free PMC article. - Systemic NF-κB-mediated inflammation promotes an aging phenotype in skeletal stem/progenitor cells.
Josephson AM, Leclerc K, Remark LH, Lopeź EM, Leucht P. Josephson AM, et al. Aging (Albany NY). 2021 May 25;13(10):13421-13429. doi: 10.18632/aging.203083. Epub 2021 May 25. Aging (Albany NY). 2021. PMID: 34035186 Free PMC article. - Relationship of Aging, Inflammation, and Skeletal Stem Cells and Their Effects on Fracture Repair.
Goodnough LH, Goodman SB. Goodnough LH, et al. Curr Osteoporos Rep. 2022 Oct;20(5):320-325. doi: 10.1007/s11914-022-00742-x. Epub 2022 Sep 21. Curr Osteoporos Rep. 2022. PMID: 36129609 Review. - Skeletal stem and progenitor cells in bone development and repair.
Trompet D, Melis S, Chagin AS, Maes C. Trompet D, et al. J Bone Miner Res. 2024 Jul 23;39(6):633-654. doi: 10.1093/jbmr/zjae069. J Bone Miner Res. 2024. PMID: 38696703 Review. - Skeletal Stem/Progenitor Cells in Periosteum and Skeletal Muscle Share a Common Molecular Response to Bone Injury.
Julien A, Perrin S, Martínez-Sarrà E, Kanagalingam A, Carvalho C, Luka M, Ménager M, Colnot C. Julien A, et al. J Bone Miner Res. 2022 Aug;37(8):1545-1561. doi: 10.1002/jbmr.4616. Epub 2022 Jun 29. J Bone Miner Res. 2022. PMID: 35652423 Free PMC article.
Cited by
- Pulse Pressure: An Emerging Therapeutic Target for Dementia.
Levin RA, Carnegie MH, Celermajer DS. Levin RA, et al. Front Neurosci. 2020 Jun 24;14:669. doi: 10.3389/fnins.2020.00669. eCollection 2020. Front Neurosci. 2020. PMID: 32670015 Free PMC article. - A Revised Perspective of Skeletal Stem Cell Biology.
Ambrosi TH, Longaker MT, Chan CKF. Ambrosi TH, et al. Front Cell Dev Biol. 2019 Sep 13;7:189. doi: 10.3389/fcell.2019.00189. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31572721 Free PMC article. Review. - Fracture Healing in the Setting of Endocrine Diseases, Aging, and Cellular Senescence.
Saul D, Khosla S. Saul D, et al. Endocr Rev. 2022 Nov 25;43(6):984-1002. doi: 10.1210/endrev/bnac008. Endocr Rev. 2022. PMID: 35182420 Free PMC article. Review. - WNT/beta-catenin signalling interrupts a senescence-induction cascade in human mesenchymal stem cells that restricts their expansion.
Lehmann J, Narcisi R, Franceschini N, Chatzivasileiou D, Boer CG, Koevoet WJLM, Putavet D, Drabek D, van Haperen R, de Keizer PLJ, van Osch GJVM, Ten Berge D. Lehmann J, et al. Cell Mol Life Sci. 2022 Jan 20;79(2):82. doi: 10.1007/s00018-021-04035-x. Cell Mol Life Sci. 2022. PMID: 35048158 Free PMC article. - Molecular Insights Into Lysyl Oxidases in Cartilage Regeneration and Rejuvenation.
Lin W, Xu L, Li G. Lin W, et al. Front Bioeng Biotechnol. 2020 Apr 30;8:359. doi: 10.3389/fbioe.2020.00359. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32426343 Free PMC article. Review.
References
- Burge R, et al. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465–475. - PubMed
- Nishida S, Endo N, Yamagiwa H, Tanizawa T, Takahashi HE. Number of osteoprogenitor cells in human bone marrow markedly decreases after skeletal maturation. J Bone Miner Metab. 1999;17:171–177. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases