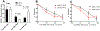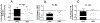Hemoglobin β93 Cysteine Is Not Required for Export of Nitric Oxide Bioactivity From the Red Blood Cell - PubMed (original) (raw)
. 2019 Jun 4;139(23):2654-2663.
doi: 10.1161/CIRCULATIONAHA.118.039284. Epub 2019 Mar 25.
Jiangning Yang 2, Andrei L Kleschyov 3 4, Zhengbing Zhuge 3, Mattias Carlström 3, John Pernow 2, Nadeem Wajih 5, T Scott Isbell 6, Joo-Yeun Oh 7 8, Pedro Cabrales 9, Amy G Tsai 9, Tim Townes 1, Daniel B Kim-Shapiro 5, Rakesh P Patel 7 8, Jon O Lundberg 3
Affiliations
- PMID: 30905171
- PMCID: PMC6546526
- DOI: 10.1161/CIRCULATIONAHA.118.039284
Hemoglobin β93 Cysteine Is Not Required for Export of Nitric Oxide Bioactivity From the Red Blood Cell
Chiao-Wang Sun et al. Circulation. 2019.
Abstract
Background: Nitrosation of a conserved cysteine residue at position 93 in the hemoglobin β chain (β93C) to form S-nitroso (SNO) hemoglobin (Hb) is claimed to be essential for export of nitric oxide (NO) bioactivity by the red blood cell (RBC) to mediate hypoxic vasodilation and cardioprotection.
Methods: To test this hypothesis, we used RBCs from mice in which the β93 cysteine had been replaced with alanine (β93A) in a number of ex vivo and in vivo models suitable for studying export of NO bioactivity.
Results: In an ex vivo model of cardiac ischemia/reperfusion injury, perfusion of a mouse heart with control RBCs (β93C) pretreated with an arginase inhibitor to facilitate export of RBC NO bioactivity improved cardiac recovery after ischemia/reperfusion injury, and the response was similar with β93A RBCs. Next, when human platelets were coincubated with RBCs and then deoxygenated in the presence of nitrite, export of NO bioactivity was detected as inhibition of ADP-induced platelet activation. This effect was the same in β93C and β93A RBCs. Moreover, vascular reactivity was tested in rodent aortas in the presence of RBCs pretreated with S-nitrosocysteine or with hemolysates or purified Hb treated with authentic NO to form nitrosyl(FeII)-Hb, the proposed precursor of SNO-Hb. SNO-RBCs or NO-treated Hb induced vasorelaxation, with no differences between β93C and β93A RBCs. Finally, hypoxic microvascular vasodilation was studied in vivo with a murine dorsal skin-fold window model. Exposure to acute systemic hypoxia caused vasodilatation, and the response was similar in β93C and β93A mice.
Conclusions: RBCs clearly have the fascinating ability to export NO bioactivity, but this occurs independently of SNO formation at the β93 cysteine of Hb.
Keywords: S-nitrosothiols; hemoglobins; nitric oxide; nitrite; nitrosation.
Conflict of interest statement
Conflict of Interest Disclosures:
JOL is a named co-inventor of patents and patent applications related to the medical uses of inorganic nitrate and nitrite. RPP and DBK-S are co-inventors on a patent for use of nitrite salts for the treatment of cardiovascular conditions. The other co-authors report no conflict of interest.
Figures
Figure 1.. Scheme of approaches used to study export of NO bioactivity by red blood cells.
Washed red blood cells (RBCs) from control mice (β93C) or mutant mice (β93A) lacking the cysteine-93 on the β-chain of hemoglobin were used in 3 model systems known to respond to export of RBC NO bioactivity: i) isolated aortic dilation or contraction in response to NO- or S-nitrosothiol treated RBCs, ii) isolated mouse hearts perfused with RBCs prior to ischemia reperfusion injury and measuring cardiac recovery, iii) RBC and nitrite-dependent inhibition of platelets activation, iv) in vivo assessment of hypoxic vasodilation.
Figure 2.. Effects of β93C and β93A RBCs on the recovery of cardiac function following ischemia-reperfusion
Hearts from wild type mice were given red blood cells (RBCs) from β93C mice incubated with vehicle (n=6) or nor-NOHA (n=5) or RBCs from β93A mice incubated with vehicle (n=5) or nor-NOHA (n=8). Nor-NOHA is an arginase inhibitor that induces export of NO bioactivity from RBCs. Data are shown as mean ± SEM. Significant differences between groups were analyzed using two-way ANOVA; **P < 0.01, ***P < 0.001. LVDP= Left Ventricular Developed Pressure.
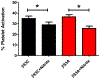
Figure 3.. β93C is not required for RBC-mediated inhibition of platelet activation by nitrite
Murine red blood cells (RBCs) containing human hemoglobin β93C and mutant of β93C to β93A were used in the platelet activation assay at 10% hematocrit under partially deoxygenated conditions. Data show mean ± SEM, n=4. P values (*) were calculated using a paired t test: p< 0.03 between β93C with and without nitrite and p< 0.03 between β93A with and without nitrite.
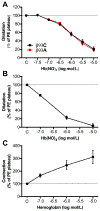
Figure 4.. Responses of mouse aortae to hemolysates from of β93C and β93A RBCs or purified hemoglobin treated with NO gas
Hemolysates from red blood cells (RBCs) of β93C or β93A mice (A) or purified human ferrohemoglobin (B) were treated with NO gas in the absence of oxygen and then tested on phenylephrine (PE) and L-NAME (1 mM, used only in A) preconstricted mouse aortae. Non-treated human ferrohemoglobin (C) was used as control. Data show mean ± SEM. n=3–6.
Figure 5:. Effects of β93C and β93A on SNO-RBC dependent vasodilation
Panel A: S-nitrosothiol (SNO) concentrations normalized to heme in β93C and β93A red blood cells (RBCs) after reaction with S-nitroso cysteine. Data show total levels and levels after fractionating RBCs into high and low molecular weight (MWt) components. Data are mean ± SEM (n=3). P-values shown were calculated by unpaired t-test. Panel B and C: Rat aortic rings were incubated at 21% or 1% O2 respectively, and vasodilation assessed in response to increasing concentrations of SNO-β93C RBCs and SNO-β93A RBCs. Data are mean ± SEM (n=3).
Figure 6:. In vivo effects of hypoxia on microvascular and hemodynamic parameters in β93C and β93A mice
β93C or β93A mice were exposed to global hypoxia and changes in arteriolar diameter (Panel A), mean arterial pressure (MAP, Panel B) and heart rate (HR, Panel C) were measured. Data show fold changes relative to normoxia and mean ± SEM (n=13–14). P value was not significant (NS) by unpaired t-test. Changes in MAP and Arterial diameter in response to hypoxia were significant in both groups of mice (p<0.001, students t test).
Comment in
- Red Blood Cell-Derived Nitric Oxide Bioactivity and Hypoxic Vasodilation.
Schmidt HHHW, Feelisch M. Schmidt HHHW, et al. Circulation. 2019 Jun 4;139(23):2664-2667. doi: 10.1161/CIRCULATIONAHA.119.040423. Epub 2019 Jun 3. Circulation. 2019. PMID: 31157996 No abstract available. - Response by Lundberg et al to Letter Regarding Article, "Hemoglobin β93 Cysteine Is Not Required for Export of Nitric Oxide Bioactivity From the Red Blood Cell".
Lundberg JO, Cabrales P, Tsai AG, Patel RP, Kim-Shapiro DB. Lundberg JO, et al. Circulation. 2019 Nov 5;140(19):e760-e761. doi: 10.1161/CIRCULATIONAHA.119.043151. Epub 2019 Nov 4. Circulation. 2019. PMID: 31682526 Free PMC article. No abstract available. - Letter by Reynolds et al Regarding Article, "Hemoglobin β93 Cysteine Is Not Required for Export of Nitric Oxide Bioactivity From the Red Blood Cell".
Reynolds JD, Premont RT, Stamler JS. Reynolds JD, et al. Circulation. 2019 Nov 5;140(19):e758-e759. doi: 10.1161/CIRCULATIONAHA.119.041389. Epub 2019 Nov 4. Circulation. 2019. PMID: 31682530 Free PMC article. No abstract available.
Similar articles
- Red Blood Cell-Mediated S-Nitrosohemoglobin-Dependent Vasodilation: Lessons Learned from a β-Globin Cys93 Knock-In Mouse.
Premont RT, Reynolds JD, Zhang R, Stamler JS. Premont RT, et al. Antioxid Redox Signal. 2021 Apr 20;34(12):936-961. doi: 10.1089/ars.2020.8153. Epub 2020 Jul 23. Antioxid Redox Signal. 2021. PMID: 32597195 Free PMC article. Review. - SNO-hemoglobin is not essential for red blood cell-dependent hypoxic vasodilation.
Isbell TS, Sun CW, Wu LC, Teng X, Vitturi DA, Branch BG, Kevil CG, Peng N, Wyss JM, Ambalavanan N, Schwiebert L, Ren J, Pawlik KM, Renfrow MB, Patel RP, Townes TM. Isbell TS, et al. Nat Med. 2008 Jul;14(7):773-7. doi: 10.1038/nm1771. Epub 2008 May 30. Nat Med. 2008. PMID: 18516054 Free PMC article. - Optimized S-nitrosohemoglobin Synthesis in Red Blood Cells to Preserve Hypoxic Vasodilation Via _β_Cys93.
Hausladen A, Qian Z, Zhang R, Premont RT, Stamler JS. Hausladen A, et al. J Pharmacol Exp Ther. 2022 Jul;382(1):1-10. doi: 10.1124/jpet.122.001194. Epub 2022 May 5. J Pharmacol Exp Ther. 2022. PMID: 35512801 Free PMC article. - Pulmonary vascular effects of red blood cells containing S-nitrosated hemoglobin.
Deem S, Kim SS, Min JH, Eveland R, Moulding J, Martyr S, Wang X, Swenson ER, Gladwin MT. Deem S, et al. Am J Physiol Heart Circ Physiol. 2004 Dec;287(6):H2561-8. doi: 10.1152/ajpheart.00310.2004. Epub 2004 Aug 5. Am J Physiol Heart Circ Physiol. 2004. PMID: 15297254 - Role of Nitric Oxide Carried by Hemoglobin in Cardiovascular Physiology: Developments on a Three-Gas Respiratory Cycle.
Premont RT, Reynolds JD, Zhang R, Stamler JS. Premont RT, et al. Circ Res. 2020 Jan 3;126(1):129-158. doi: 10.1161/CIRCRESAHA.119.315626. Epub 2019 Oct 8. Circ Res. 2020. PMID: 31590598 Free PMC article. Review.
Cited by
- Metabolic landscape in venous thrombosis: insights into molecular biology and therapeutic implications.
Cao Z, Jiang X, He Y, Zheng X. Cao Z, et al. Ann Med. 2024 Dec;56(1):2401112. doi: 10.1080/07853890.2024.2401112. Epub 2024 Sep 19. Ann Med. 2024. PMID: 39297312 Free PMC article. Review. - Nitric Oxide Production and Regulation in the Teleost Cardiovascular System.
Giordano D, Verde C, Corti P. Giordano D, et al. Antioxidants (Basel). 2022 May 12;11(5):957. doi: 10.3390/antiox11050957. Antioxidants (Basel). 2022. PMID: 35624821 Free PMC article. Review. - The Effects of Different Signaling Pathways in Adenylyl Cyclase Stimulation on Red Blood Cells Deformability.
Semenov AN, Shirshin EA, Muravyov AV, Priezzhev AV. Semenov AN, et al. Front Physiol. 2019 Aug 14;10:923. doi: 10.3389/fphys.2019.00923. eCollection 2019. Front Physiol. 2019. PMID: 31474870 Free PMC article. - Response by Lundberg et al to Letter Regarding Article, "Hemoglobin β93 Cysteine Is Not Required for Export of Nitric Oxide Bioactivity From the Red Blood Cell".
Lundberg JO, Cabrales P, Tsai AG, Patel RP, Kim-Shapiro DB. Lundberg JO, et al. Circulation. 2019 Nov 5;140(19):e760-e761. doi: 10.1161/CIRCULATIONAHA.119.043151. Epub 2019 Nov 4. Circulation. 2019. PMID: 31682526 Free PMC article. No abstract available. - How Nitric Oxide Hindered the Search for Hemoglobin-Based Oxygen Carriers as Human Blood Substitutes.
Samaja M, Malavalli A, Vandegriff KD. Samaja M, et al. Int J Mol Sci. 2023 Oct 4;24(19):14902. doi: 10.3390/ijms241914902. Int J Mol Sci. 2023. PMID: 37834350 Free PMC article. Review.
References
- Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: A dynamic activity of blood involved in vascular control. Nature. 1996;380:221–226. - PubMed
- McMahon TJ, Moon RE, Luschinger BP, Carraway MS, Stone AE, Stolp BW, Gow AJ, Pawloski JR, Watke P, Singel DJ, Piantadosi CA, Stamler JS. Nitric oxide in the human respiratory cycle. Nat Med. 2002;8:711–717. - PubMed
- Pawloski JR, Hess DT, Stamler JS. Export by red blood cells of nitric oxide bioactivity. Nature. 2001;409:622–626. - PubMed
- Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, Gernert K, Piantadosi CA. Blood flow regulation by s-nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276:2034–2037. - PubMed
- Doctor A, Stamler JS. Nitric oxide transport in blood: A third gas in the respiratory cycle. Comp Physiol. 2011;1:541–568. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL058091/HL/NHLBI NIH HHS/United States
- R37 HL058091/HL/NHLBI NIH HHS/United States
- R01 HL098032/HL/NHLBI NIH HHS/United States
- R01 HL138116/HL/NHLBI NIH HHS/United States
- R01 HL126945/HL/NHLBI NIH HHS/United States
- R01 HL092624/HL/NHLBI NIH HHS/United States
- R29 HL058091/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases