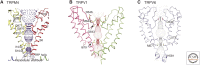Transient Receptor Potential Channels and Calcium Signaling - PubMed (original) (raw)
Review
Transient Receptor Potential Channels and Calcium Signaling
Laura Vangeel et al. Cold Spring Harb Perspect Biol. 2019.
Abstract
Transient receptor potential (TRP) cation channels play diverse roles in cellular Ca2+ signaling. First, as Ca2+-permeable channels that respond to a variety of stimuli, TRP channels can directly initiate cellular Ca2+ signals. Second, as nonselective cation channels, TRP channel activation leads to membrane depolarization, influencing Ca2+ influx via voltage-gated and store-operated Ca2+ channels. Finally, Ca2+ modulates the activity of most TRP channels, allowing them to function as molecular effectors downstream of intracellular Ca2+ signals. Whereas the TRP channel field has long been devoid of detailed channel structures, recent advances, particularly in cryo-electron microscopy-based structural approaches, have yielded a flurry of TRP channel structures, including members from all seven subfamilies. These structures, in conjunction with mutagenesis-based functional approaches, provided important new insights into the mechanisms whereby TRP channels permeate and sense Ca2+ These insights will be highly instrumental in the rational design of novel treatments for the multitude of TRP channel-related diseases.
Copyright © 2019 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
Figure 1.
Ca2+ permeability of transient receptor potential (TRP) channels. On a logarithmic scale, these are examples of the indicated mammalian TRP channels illustrating the range of relative Ca2+ permeabilities (based on reversal potential measurements). Note that values are indicative, as the obtained values can vary significantly depending on cellular environment and experimental conditions.
Figure 2.
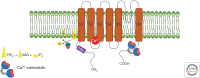
Cartoon illustrating three general mechanisms of Ca2+-dependent regulation of transient receptor potential (TRP) channel activity. (Left) Ca2+ (red spheres) can cause a decrease in the plasma membrane PIP2 levels via Ca2+-activated phospholipase C. This decrease is sensed by PIP2-binding domains (PBDs, purple), which are found at various locations in the cytosolic part of TRP channels. (Center) Ca2+ can bind directly to activate TRP channels, for instance, via residues in the S2-S3 region of TRPM channels. (Right) Ca2+ can influence TRP channel activity via calmodulin (CaM, blue), for example, via Ca2+-CaM binding in the carboxyl terminus of TRPV channels.
Figure 3.
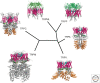
Cladogram of mammalian transient receptor potential (TRP) channel subfamilies and their structures. A representative structure of a member of each subfamily is illustrated: hTRML1 (PDB ID: 5WJ9), hTRPP2 (5K47), hTRPA1 (3J9P), rTRPV1 (3J5P), hTRPM4 (6BQR), and hTRPC3 (6CUD). Specific domains are indicated with the following color code: pink = transmembrane domains (S1–S6), light blue = pore region, dark blue = selectivity filter, orange = ankyrin repeat domain, dark green = TRP domain, yellow = TRP box, and lime = S1–S2 extracellular domain (luminal domain [TRPML] and polycystin domain [TRPP]).
Figure 4.
Structure of transient receptor potential (TRP) channel pores. Comparison of the pore domains of TRPM4 (left, Ca2+-impermeable), TRPV1 (middle, Ca2+-permeable, nonselective), and TRPV6 (right, Ca2+selective). Structures are created from data in Winkler et al. (2017) (TRPM4), Liao et al. (2013) (TRPV1-apo state), and Hughes et al. (2018a) (TRPV6) (see text for more details).
Figure 5.
Structural elements underlying Ca2+-dependent regulation of transient receptor potential (TRP) channels. (Left) Interaction between phosphatidylinositol bisphosphate (PIP2) and the PIP2-binding pocket in TRPV5, which is formed by residues from the amino terminus (Arg302, Arg305), the S4–S5 linker (Lys484), and the S6-helix (Arg584). PIP2 is shown in red, interacting amino acids in salmon. (Center) A Ca2+ ion (red) interacting with TRPM4 via residues Glu828, Gln831 (from S2), and Asn865 and Asp868 (from S3). (Right) Ca2+ ions (red) bound to calmodulin (pink), interacting with the carboxyl terminus of TRPV5 via an interaction with His699, Trp702, and Thr709 (salmon).
Similar articles
- Regulation and Role of Store-Operated Ca2+ Entry in Cellular Proliferation.
Hodeify R, Yu F, Courjaret R, Nader N, Dib M, Sun L, Adap E, Hubrack S, Machaca K. Hodeify R, et al. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 12. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 12. PMID: 30299656 Free Books & Documents. Review. - Determining the Crystal Structure of TRPV6.
Saotome K, Singh AK, Sobolevsky AI. Saotome K, et al. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 14. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 14. PMID: 30299652 Free Books & Documents. Review. - Calcium-permeable ion channels in the kidney.
Zhou Y, Greka A. Zhou Y, et al. Am J Physiol Renal Physiol. 2016 Jun 1;310(11):F1157-67. doi: 10.1152/ajprenal.00117.2016. Epub 2016 Mar 30. Am J Physiol Renal Physiol. 2016. PMID: 27029425 Free PMC article. Review. - Transient receptor potential channels in the vasculature.
Earley S, Brayden JE. Earley S, et al. Physiol Rev. 2015 Apr;95(2):645-90. doi: 10.1152/physrev.00026.2014. Physiol Rev. 2015. PMID: 25834234 Free PMC article. Review. - Structural biology of thermoTRPV channels.
Yuan P. Yuan P. Cell Calcium. 2019 Dec;84:102106. doi: 10.1016/j.ceca.2019.102106. Epub 2019 Nov 1. Cell Calcium. 2019. PMID: 31726322 Free PMC article. Review.
Cited by
- The Extremely-Low-Frequency Electromagnetic Field Affects Apoptosis and Oxidative-Stress-Related Genes and Proteins in the Porcine Endometrium-An In Vitro Study.
Wydorski PJ, Zmijewska A, Franczak A. Wydorski PJ, et al. Int J Mol Sci. 2024 Jun 25;25(13):6931. doi: 10.3390/ijms25136931. Int J Mol Sci. 2024. PMID: 39000040 Free PMC article. - S-acylation of Ca2+ transport proteins in cancer.
Kouba S, Demaurex N. Kouba S, et al. Chronic Dis Transl Med. 2024 Aug 14;10(4):263-280. doi: 10.1002/cdt3.146. eCollection 2024 Dec. Chronic Dis Transl Med. 2024. PMID: 39429488 Free PMC article. Review. - Transient Receptor Potential (TRP) and Thermoregulation in Animals: Structural Biology and Neurophysiological Aspects.
Lezama-García K, Mota-Rojas D, Pereira AMF, Martínez-Burnes J, Ghezzi M, Domínguez A, Gómez J, de Mira Geraldo A, Lendez P, Hernández-Ávalos I, Falcón I, Olmos-Hernández A, Wang D. Lezama-García K, et al. Animals (Basel). 2022 Jan 2;12(1):106. doi: 10.3390/ani12010106. Animals (Basel). 2022. PMID: 35011212 Free PMC article. Review. - An inactivating human TRPC6 channel mutation without focal segmental glomerulosclerosis.
Batool L, Hariharan K, Xu Y, Kaßmann M, Tsvetkov D, Gohlke BO, Kaden S, Gossen M, Nürnberg B, Kurtz A, Gollasch M. Batool L, et al. Cell Mol Life Sci. 2023 Aug 24;80(9):265. doi: 10.1007/s00018-023-04901-w. Cell Mol Life Sci. 2023. PMID: 37615749 Free PMC article. - Low-Intensity Extracorporeal Shock Wave Therapy Ameliorates Detrusor Hyperactivity with Impaired Contractility via Transient Potential Vanilloid Channels: A Rat Model for Ovarian Hormone Deficiency.
Chueh KS, Juan TJ, Lu JH, Wu BN, Lin RJ, Mao JW, Lin HY, Chuang SM, Chang CY, Shen MC, Sun TW, Juan YS. Chueh KS, et al. Int J Mol Sci. 2024 Apr 30;25(9):4927. doi: 10.3390/ijms25094927. Int J Mol Sci. 2024. PMID: 38732143 Free PMC article.
References
- Arias-Darraz L, Cabezas D, Colenso CK, Alegría-Arcos M, Bravo-Moraga F, Varas-Concha I, Almonacid DE, Madrid R, Brauchi S. 2015. A transient receptor potential ion channel in Chlamydomonas shares key features with sensory transduction-associated TRP channels in mammals. Plant Cell 27: 177–188. 10.1105/tpc.114.131862 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous