How rapid advances in imaging are defining the future of precision radiation oncology - PubMed (original) (raw)
Review
How rapid advances in imaging are defining the future of precision radiation oncology
Laura Beaton et al. Br J Cancer. 2019 Apr.
Abstract
Imaging has an essential role in the planning and delivery of radiotherapy. Recent advances in imaging have led to the development of advanced radiotherapy techniques-including image-guided radiotherapy, intensity-modulated radiotherapy, stereotactic body radiotherapy and proton beam therapy. The optimal use of imaging might enable higher doses of radiation to be delivered to the tumour, while sparing normal surrounding tissues. In this article, we review how the integration of existing and novel forms of computed tomography, magnetic resonance imaging and positron emission tomography have transformed tumour delineation in the radiotherapy planning process, and how these advances have the potential to allow a more individualised approach to the cancer therapy. Recent data suggest that imaging biomarkers that assess underlying tumour heterogeneity can identify areas within a tumour that are at higher risk of radio-resistance, and therefore potentially allow for biologically focussed dose escalation. The rapidly evolving concept of adaptive radiotherapy, including artificial intelligence, requires imaging during treatment to be used to modify radiotherapy on a daily basis. These advances have the potential to improve clinical outcomes and reduce radiation-related long-term toxicities. We outline how recent technological advances in both imaging and radiotherapy delivery can be combined to shape the future of precision radiation oncology.
Conflict of interest statement
R.A.S. is funded by the NIHR University College London Hospitals Biomedical Research Centre, Cancer Research UK (Grant A8971 CRUK/07/030) and research grants from Sirtex Medical and BTG plc. R.A.S. declares consultancy with Affidea, Astra Zeneca, Boston Scientific, BTG, Cancer Research Technology, DeepMind, Eisai, Sirtex, Terumo and Varian. S.B. declares consultancy with Angiodynamics UK Ltd. The remaining authors declare no competing interests.
Figures
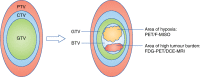
Fig. 1
Target volume definitions and the concept of the biological target volume. At present, target volume (tumour) delineation is characterised by the concepts of gross tumour volume (GTV), clinical target volume (CTV) and planning target volumes (PTV). Information from functional imaging may provide additional information for defining the ‘biological target volume’ (BTV)—a subvolume of the tumour that may indicate underlying radio-resistance. For example, hypoxic areas may be derived from PET-18F-misonidazole (F-MISO) imaging and high tumour burden from 18-F-fluorodeoxyglucose positron emission tomography (FDG-PET)/CT or dynamic contract enhanced magnetic resonance imaging (DCE-MRI). A higher dose of radiation can then be delivered to the BTV (rather that the whole tumour) in a process known as dose painting (image adapted from Ling et al.)
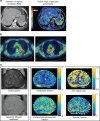
Fig. 2
Examples of functional imaging techniques. a Functional CT technique for measuring perfusion parameters (in tumours and normal liver). Perfusion CT images from a patient with a large liver metastasis from colorectal cancer. (Left panel) Aorta (marked 1), liver tumour (marked 2) and normal tissue (marked 3) delineated as regions of interest (ROIs); (right panel) Colour maps of perfusion parameters within ROIs. Red indicates higher blood flow. b 18-F‐fluoromisonidazole uptake in advanced stage non‐small cell lung cancer. Two examples of F-MISO PET/CT images on an SUV scale 0–3 for two different patients with non-small cell lung cancer. Images courtesy of Geoff Higgins, Oxford Institute for Radiation Oncology. c ADC and VERDICT magnetic resonance images from a patient with prostate cancer. MRI images from a patient with prostate cancer (biopsy confirmed). (Left panel) Axial T2-weighted image and ADC map from standard mpMRI; and (right panel), vascular Extracellular Restricted Diffusion for Cytometry in Tumours (VERDICT) volume fraction maps of the intracellular, the extracellular-extravascular and the vascular components, and the cell radius index map. Images courtesy of Laura Panagiotaki, University College London. ADC apparent diffusion coefficient, mpMRI multi-parametric magnetic resonance imaging, VERDICT Vascular Extracellular Restricted Diffusion for Cytometry in Tumours
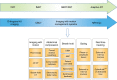
Fig. 3
Overview of advances in radiotherapy techniques and image-guided radiotherapy (IGRT) over time. Recent advances in imaging techniques have enabled tumours to be more accurately delineated for radiotherapy treatment and for image guidance during therapy. The advent of CBCT and motion management systems has allowed radiotherapy to become more highly conformal, and high ablative doses can be safely delivered with sparing of surrounding normal tissues. The development of the MR Linac takes this one step further by offering the prospect of real-time imaging using MRI to permit daily adaptive radiotherapy. CRT conformal radiotherapy; IMRT intensity-modulated radiotherapy; SBRT stereotactic body radiotherapy; PBT proton beam therapy; CBCT cone beam CT**;** DIBH deep-inspiration breath hold; ABC active-breathing control
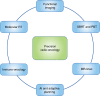
Fig. 4
The future of precision radiation oncology. The future of precision radiation oncology will incorporate advanced radiotherapy techniques with functional imaging that will allow for biological dose optimisation. Novel biologically targeted radiopharmaceuticals will enable selective delivery of internal radiation, termed molecular radiotherapy. Immune modulators will be used in combination with different radiotherapy techniques in the field of immuno radio-oncology to take advantage of the synergy between these two modalities of treatment. Artificial intelligence (AI) will assist with the adaptive planning process, allowing a new radiation plan to be created, based on imaging obtained on the day of treatment. SBRT stereotactic body radiotherapy, PBT proton beam therapy, CT computerised tomography, MR Linac magnetic resonance linear accelerator
Similar articles
- [Image-guided radiotherapy].
de Crevoisier R, Isambert A, Lisbona A, Bodez V, Marguet M, Lafay F, Remonnay R, Lagrange JL. de Crevoisier R, et al. Cancer Radiother. 2007 Nov;11(6-7):296-304. doi: 10.1016/j.canrad.2007.08.002. Epub 2007 Sep 21. Cancer Radiother. 2007. PMID: 17889585 Review. French. - Technological evolution of radiation treatment: Implications for clinical applications.
Pacelli R, Caroprese M, Palma G, Oliviero C, Clemente S, Cella L, Conson M. Pacelli R, et al. Semin Oncol. 2019 Jun;46(3):193-201. doi: 10.1053/j.seminoncol.2019.07.004. Epub 2019 Jul 30. Semin Oncol. 2019. PMID: 31395286 Review. - Current State of Image Guidance in Radiation Oncology: Implications for PTV Margin Expansion and Adaptive Therapy.
Zou W, Dong L, Kevin Teo BK. Zou W, et al. Semin Radiat Oncol. 2018 Jun;28(3):238-247. doi: 10.1016/j.semradonc.2018.02.008. Semin Radiat Oncol. 2018. PMID: 29933883 Review. - Radiomics for liver tumours.
Dreher C, Linde P, Boda-Heggemann J, Baessler B. Dreher C, et al. Strahlenther Onkol. 2020 Oct;196(10):888-899. doi: 10.1007/s00066-020-01615-x. Epub 2020 Apr 15. Strahlenther Onkol. 2020. PMID: 32296901 Free PMC article. Review. - Technology for Innovation in Radiation Oncology.
Chetty IJ, Martel MK, Jaffray DA, Benedict SH, Hahn SM, Berbeco R, Deye J, Jeraj R, Kavanagh B, Krishnan S, Lee N, Low DA, Mankoff D, Marks LB, Ollendorf D, Paganetti H, Ross B, Siochi RA, Timmerman RD, Wong JW. Chetty IJ, et al. Int J Radiat Oncol Biol Phys. 2015 Nov 1;93(3):485-92. doi: 10.1016/j.ijrobp.2015.07.007. Epub 2015 Jul 11. Int J Radiat Oncol Biol Phys. 2015. PMID: 26460989 Free PMC article.
Cited by
- Application of H2N-Fe3O4 Nanoparticles for Prostate Cancer Magnetic Resonance Imaging in an Animal Model.
Blasiak B, MacDonald D, Jasiński K, Cheng FY, Tomanek B. Blasiak B, et al. Int J Mol Sci. 2024 Sep 26;25(19):10334. doi: 10.3390/ijms251910334. Int J Mol Sci. 2024. PMID: 39408664 Free PMC article. - Clinical research progress of callisperes® of drug-loaded microsphere arterial chemoembolisation in the treatment of solid tumors.
Wang Q, Zhu L, Sheng Q. Wang Q, et al. Discov Oncol. 2024 May 13;15(1):161. doi: 10.1007/s12672-024-01030-z. Discov Oncol. 2024. PMID: 38739205 Free PMC article. Review. - Systematic Review and Meta-analysis of the Association Between Radiation Therapy Treatment Volume and Patient Outcomes.
Kyaw JYA, Rendall A, Gillespie EF, Roques T, Court L, Lievens Y, Tree AC, Frampton C, Aggarwal A. Kyaw JYA, et al. Int J Radiat Oncol Biol Phys. 2023 Dec 1;117(5):1063-1086. doi: 10.1016/j.ijrobp.2023.02.048. Epub 2023 May 25. Int J Radiat Oncol Biol Phys. 2023. PMID: 37227363 Free PMC article. - Trimodality PET/CT/MRI and Radiotherapy: A Mini-Review.
Decazes P, Hinault P, Veresezan O, Thureau S, Gouel P, Vera P. Decazes P, et al. Front Oncol. 2021 Feb 4;10:614008. doi: 10.3389/fonc.2020.614008. eCollection 2020. Front Oncol. 2021. PMID: 33614497 Free PMC article. Review. - Empowering PET: harnessing deep learning for improved clinical insight.
Artesani A, Bruno A, Gelardi F, Chiti A. Artesani A, et al. Eur Radiol Exp. 2024 Feb 7;8(1):17. doi: 10.1186/s41747-023-00413-1. Eur Radiol Exp. 2024. PMID: 38321340 Free PMC article. Review.
References
- Ringborg U, Bergqvist D, Brorsson B, Cavallin-ståhl E, Ceberg J, Einhorn N, et al. The Swedish Council on Technology Assessment in Health Care (SBU) systematic overview of radiotherapy for cancer including a prospective survey of radiotherapy practice in Sweden 2001—summary and conclusions. Acta Oncol. 2003;42:357–365. - PubMed
- Sharma RA, Plummer R, Stock JK, Greenhalgh TA, Ataman O, Kelly S, et al. Clinical development of new drug-radiotherapy combinations. Nat. Rev. Clin. Oncol. 2016;13:627–642. - PubMed
- Chow E, Zeng L, Salvo N, Dennis K, Tsao M, Lutz S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin. Oncol. 2012;24:112–124. - PubMed
- Tree AC, Khoo VS, Eeles RA, Ahmed M, Dearnaley DP, Hawkins MA, et al. Stereotactic body radiotherapy for oligometastases. Lancet Oncol. 2013;14:e28–e37. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources