Energy metabolism in anaerobic eukaryotes and Earth's late oxygenation - PubMed (original) (raw)
Review
Energy metabolism in anaerobic eukaryotes and Earth's late oxygenation
Verena Zimorski et al. Free Radic Biol Med. 2019.
Abstract
Eukaryotes arose about 1.6 billion years ago, at a time when oxygen levels were still very low on Earth, both in the atmosphere and in the ocean. According to newer geochemical data, oxygen rose to approximately its present atmospheric levels very late in evolution, perhaps as late as the origin of land plants (only about 450 million years ago). It is therefore natural that many lineages of eukaryotes harbor, and use, enzymes for oxygen-independent energy metabolism. This paper provides a concise overview of anaerobic energy metabolism in eukaryotes with a focus on anaerobic energy metabolism in mitochondria. We also address the widespread assumption that oxygen improves the overall energetic state of a cell. While it is true that ATP yield from glucose or amino acids is increased in the presence of oxygen, it is also true that the synthesis of biomass costs thirteen times more energy per cell in the presence of oxygen than in anoxic conditions. This is because in the reaction of cellular biomass with O2, the equilibrium lies very far on the side of CO2. The absence of oxygen offers energetic benefits of the same magnitude as the presence of oxygen. Anaerobic and low oxygen environments are ancient. During evolution, some eukaryotes have specialized to life in permanently oxic environments (life on land), other eukaryotes have remained specialized to low oxygen habitats. We suggest that the Km of mitochondrial cytochrome c oxidase of 0.1-10 μM for O2, which corresponds to about 0.04%-4% (avg. 0.4%) of present atmospheric O2 levels, reflects environmental O2 concentrations that existed at the time that the eukaryotes arose.
Keywords: Chlamydomonas; Earth history; Euglena; Eukaryote anaerobes; Great oxidation event; Hydrogenosomes; Mitosomes.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Graphical abstract
Fig. 1
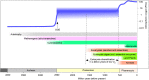
Summary of oxygen accumulation of earth history. Data for oxygen levels from Ref. [17], for the emergence of autotrophy from Ref. [1], of methanogens from Ref. [3], of cyanobacteria from Ref. [20], of animals from Ref. [24], of eukaryote anaerobes from Refs. [47,95], of eukaryote algae from Ref. [21], of algal anaerobic enzymes from Ref. [61], of land plants from Ref. [13], and for the eukaryote age from Refs. [8,30]. PAL: percent of present atmospheric level. GOE: great oxidation event. The GOE marks the appearance of continuous atmospheric O2 in the geochemical record [17,26,27,[30], [31], [32], [33]].
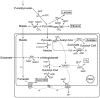
Fig. 2
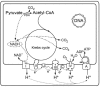
Generalized metabolic scheme of pyruvate oxidation and oxidative phosphorylation in a typical oxygen-respiring mitochondrion, for example, from rat liver. The map is redrawn after [47]. I to IV, respiratory complexes I to IV; A, ATPase; C, cytochrome c; PDH, pyruvate dehydrogenase complex; U, ubiquinone.
Fig. 3
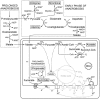
Major pathways of the facultative anaerobic energy metabolism in mitochondria of the mussel Mytilus edulis. The map is redrawn after [47]. Living attached to hard substrates, like rocks, in intertidal habitats, the bivalve has to face anaerobiosis periodically. Oxygen-independent cytosolic energy metabolism produces ATP via substrate-level phosphorylation accompanied by the formation of various end-products, including octopine, strombine, and alanine [128], which are boxed here. Under conditions of prolonged anaerobiosis, propionate is preferentially formed instead of succinate in mitochondria. Fumarate reduction is electron transfer chain coupled, and rhodoquinone serves as an electron donor to fumarate reductase. I to IV, respiratory complexes I to IV; A, ATPase; ALT, alanine aminotransferase; ASCT, acetate:succinate CoA transferase (subfamily 1B); AST, aspartate aminotransferase; C, cytochrome c; FH fumarase; FR, fumarate reductase; MDH, malate dehydrogenase; ME, malic enzyme; PDH, pyruvate dehydrogenase complex; ODH, octopine dehydrogenase; PEP-CK, phosphoenolpyruvate carboxykinase (ATP-dependent); PK, pyruvate kinase; R, rhodoquinone; SCS, succinyl-CoA synthetase; SDH, strombine dehydrogenase; U, ubiquinone.
Fig. 4
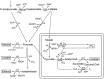
Major pathways of the anaerobic, molecular hydrogen-producing, fermentative metabolism in hydrogenosomes of the flagellated protist Trichomonas vaginalis. The map is redrawn after [47]. Hydrogenosomal pyruvate breakdown involves pyruvate:ferredoxin oxidoreductase and functional 51-kDa and 24-kDa subunits of the NADH dehydrogenase module in complex I, which reoxidize NADH stemming from malate oxidation [52,139,144]. The 51-kDa and 24-kDa subunits of mitochondrial complex I function in association with [Fe]-HYD in Trichomonas [144]. Additional major end-products of cytosolic fermentations in T. vaginalis include alanine, lactate, ethanol, and glycerol [53]. End-products are boxed. ADH, alcohol dehydrogenase (NADPH-dependent); ALT, alanine aminotransferase; ASCT, acetate:succinate CoA transferase (subfamily 1C); 24 kDa/51 kDa, 24-kDa and 51-kDa subunits of the NADH dehydrogenase module of complex I; Fd, ferredoxin; HYD, hydrogenase; LDH, lactate dehydrogenase; MDH, malate dehydrogenase; ME, malic enzyme; PDC, pyruvate decarboxylase; PEP-CK, phosphoenolpyruvate carboxykinase (GTP-dependent); PFO, pyruvate:ferredoxin oxidoreductase; PK, pyruvate kinase; SCS, succinyl-CoA synthetase.
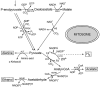
Fig. 5
Tentative map of major pathways of energy metabolism in hydrogen-producing mitochondria of the anaerobic ciliate Nyctotherus ovalis. The map is redrawn after [47]. The incomplete Krebs cycle is likely used in the reductive direction [211]. A proton gradient is generated, probably by a functional respiratory complex I, which passes the electrons from the NADH pool through rhodoquinone to complex II, acting as fumarate reductase synthesizing succinate [208]. Redox balance is also achieved with the help of hydrogenase, releasing molecular hydrogen [113]. ATP can be synthesized by substrate-level phosphorylation, producing acetate. I, respiratory complex I; II, fumarate reductase/succinate dehydrogenase; ADH, alcohol dehydrogenase (NADH-dependent); ALT, alanine aminotransferase; ASCT, acetate:succinate CoA transferase subfamily 1A; FH fumarase; GDH, glutamate dehydrogenase; HYD, hydrogenase; KGDH, alpha-ketoglutarate dehydrogenase; LDH, lactate dehydrogenase; ME, malic enzyme; R, rhodoquinone; PDC, pyruvate decarboxylase; PDH, pyruvate dehydrogenase; PK, pyruvate kinase; SCS, succinyl-CoA synthetase.
Fig. 6
Major pathways of the energy metabolism in the parasite Giardia intestinalis. The map is redrawn after [47]. Giardia mitosomes are not directly involved in energy metabolism but are involved in FeS cluster biogenesis [111]. The typically hydrogenosomal (and sometimes mitochondrial) enzymes PFO and [Fe]-HYD have been recompartmentalized to the cytosol during evolution. Molecular hydrogen is produced under strictly anoxic conditions [159], as indicated by the dashed line. ACS, acetyl-CoA synthetase (ADP-forming); ADHE, alcohol dehydrogenase E; ALT, alanine aminotransferase; Fd, ferredoxin; HYD, hydrogenase; MDH, malate dehydrogenase; ME, malic enzyme; PEP-CK, phosphoenolpyruvate carboxykinase (GTP-dependent); PFO, pyruvate:ferredoxin oxidoreductase; PPDK, pyruvate:orthophosphate dikinase; PK, pyruvate kinase.
Similar articles
- Biochemistry and evolution of anaerobic energy metabolism in eukaryotes.
Müller M, Mentel M, van Hellemond JJ, Henze K, Woehle C, Gould SB, Yu RY, van der Giezen M, Tielens AG, Martin WF. Müller M, et al. Microbiol Mol Biol Rev. 2012 Jun;76(2):444-95. doi: 10.1128/MMBR.05024-11. Microbiol Mol Biol Rev. 2012. PMID: 22688819 Free PMC article. Review. - Energy metabolism among eukaryotic anaerobes in light of Proterozoic ocean chemistry.
Mentel M, Martin W. Mentel M, et al. Philos Trans R Soc Lond B Biol Sci. 2008 Aug 27;363(1504):2717-29. doi: 10.1098/rstb.2008.0031. Philos Trans R Soc Lond B Biol Sci. 2008. PMID: 18468979 Free PMC article. Review. - The rise of oxygen and complex life.
Van Der Giezen M, Lenton TM. Van Der Giezen M, et al. J Eukaryot Microbiol. 2012 Mar-Apr;59(2):111-3. doi: 10.1111/j.1550-7408.2011.00605.x. Epub 2012 Jan 30. J Eukaryot Microbiol. 2012. PMID: 22288602 - Single eubacterial origin of eukaryotic sulfide:quinone oxidoreductase, a mitochondrial enzyme conserved from the early evolution of eukaryotes during anoxic and sulfidic times.
Theissen U, Hoffmeister M, Grieshaber M, Martin W. Theissen U, et al. Mol Biol Evol. 2003 Sep;20(9):1564-74. doi: 10.1093/molbev/msg174. Epub 2003 Jun 27. Mol Biol Evol. 2003. PMID: 12832624 - Physiology, anaerobes, and the origin of mitosing cells 50 years on.
Martin WF. Martin WF. J Theor Biol. 2017 Dec 7;434:2-10. doi: 10.1016/j.jtbi.2017.01.004. Epub 2017 Jan 11. J Theor Biol. 2017. PMID: 28087421 Review.
Cited by
- A reassessment of the "hard-steps" model for the evolution of intelligent life.
Mills DB, Macalady JL, Frank A, Wright JT. Mills DB, et al. Sci Adv. 2025 Feb 14;11(7):eads5698. doi: 10.1126/sciadv.ads5698. Epub 2025 Feb 14. Sci Adv. 2025. PMID: 39951518 Free PMC article. Review. - The Peptidyl Transferase Center: a Window to the Past.
Tirumalai MR, Rivas M, Tran Q, Fox GE. Tirumalai MR, et al. Microbiol Mol Biol Rev. 2021 Dec 15;85(4):e0010421. doi: 10.1128/MMBR.00104-21. Epub 2021 Nov 10. Microbiol Mol Biol Rev. 2021. PMID: 34756086 Free PMC article. - The Roles of Mitochondria in Human Being's Life and Aging.
Indo HP, Chatatikun M, Nakanishi I, Matsumoto KI, Imai M, Kawakami F, Kubo M, Abe H, Ichikawa H, Yonei Y, Beppu HJ, Minamiyama Y, Kanekura T, Ichikawa T, Phongphithakchai A, Udomwech L, Sukati S, Charong N, Somsak V, Tangpong J, Nomura S, Majima HJ. Indo HP, et al. Biomolecules. 2024 Oct 17;14(10):1317. doi: 10.3390/biom14101317. Biomolecules. 2024. PMID: 39456251 Free PMC article. Review. - The Oxidative Paradox in Low Oxygen Stress in Plants.
Pucciariello C, Perata P. Pucciariello C, et al. Antioxidants (Basel). 2021 Feb 23;10(2):332. doi: 10.3390/antiox10020332. Antioxidants (Basel). 2021. PMID: 33672303 Free PMC article. Review. - Old genes in new places: A taxon-rich analysis of interdomain lateral gene transfer events.
Cote-L'Heureux A, Maurer-Alcalá XX, Katz LA. Cote-L'Heureux A, et al. PLoS Genet. 2022 Jun 22;18(6):e1010239. doi: 10.1371/journal.pgen.1010239. eCollection 2022 Jun. PLoS Genet. 2022. PMID: 35731825 Free PMC article.
References
- Tashiro T., Ishida A., Hori M., Igisu M. Early trace of life from 3.95 Ga sedimentary rocks in Labrador, Canada. Nature. 2017;549:516–518. - PubMed
- Arndt N., Nisbet E. Processes on the young Earth and the habitats of early life. Annu. Rev. Earth Planet Sci. 2012;40:521–549.
- Ueno Y., Yamada K., Yoshida N., Maruyama S. Evidence from fluid inclusions for microbial methanogenesis in the early Archaean era. Nature. 2006;440:516–519. - PubMed
- Fischer W.W., Hemp J., Valentine J.S. How did life survive Earth's great oxygenation? Curr. Opin. Chem. Biol. 2016;31:166–178. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources