How cells fuse - PubMed (original) (raw)
Review
. 2019 May 6;218(5):1436-1451.
doi: 10.1083/jcb.201901017. Epub 2019 Apr 1.
Affiliations
- PMID: 30936162
- PMCID: PMC6504885
- DOI: 10.1083/jcb.201901017
Review
How cells fuse
Nicolas G Brukman et al. J Cell Biol. 2019.
Abstract
Cell-cell fusion remains the least understood type of membrane fusion process. However, the last few years have brought about major advances in understanding fusion between gametes, myoblasts, macrophages, trophoblasts, epithelial, cancer, and other cells in normal development and in diseases. While different cell fusion processes appear to proceed via similar membrane rearrangements, proteins that have been identified as necessary and sufficient for cell fusion (fusogens) use diverse mechanisms. Some fusions are controlled by a single fusogen; other fusions depend on several proteins that either work together throughout the fusion pathway or drive distinct stages. Furthermore, some fusions require fusogens to be present on both fusing membranes, and in other fusions, fusogens have to be on only one of the membranes. Remarkably, some of the proteins that fuse cells also sculpt single cells, repair neurons, promote scission of endocytic vesicles, and seal phagosomes. In this review, we discuss the properties and diversity of the known proteins mediating cell-cell fusion and highlight their different working mechanisms in various contexts.
© 2019 Brukman et al.
Figures
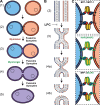
Figure 1.
Mechanisms of cell–cell fusion. (A) The pathway of cell–cell fusion. Ready-to-fuse cells (1) recognize and closely appose each other (2) and undergo hemifusion (3), i.e., the merger of the outer monolayers of two membrane bilayers, allowing redistribution of the lipid markers between the cells (note that both distal monolayers of the membranes and cell contents remain distinct). Opening of a fusion pore in the hemifusion structure allows the mixing of the cytoplasmic contents (4), and pore expansion completes joining of two cells into one (5). While Myomaker/Myomerger, syncytins, and fusexins seem to be for now the only proteins necessary for specific fusion processes, they are most likely working with other players, some of which, especially for myoblasts, are already identified. Fusexins and syncytins mediate all the stages of the fusion process; in contrast, Myomaker is required for an early stage involving the transition to hemifusion, while Myomerger is required for a later stage between hemifusion and opening of fusion pores (see the main text). (B) Schematic representation of the lipid rearrangements during the events explained in A. LPC blocks hemifusion by inhibiting the bending of the contacting monolayers (Chernomordik and Kozlov, 2003). (C) Inset from A 2: Protein fusogens are necessary to overcome the energetic barriers of hemifusion and opening and expansion of the fusion pore. Examples display bilateral and homotypic fusions mediated by C. elegans EFF-1 (upper panel) and Arabidopsis HAP2 (middle panel) as well as a bilateral and heterotypic fusion between them (lower panel; Valansi et al., 2017).
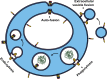
Figure 2.
Alternative functions for cell–cell fusogens. Membrane remodeling activity of EFF-1 and AFF-1 proteins is not limited to mediating cell–cell fusion events. Auto-fusion: a single cell fuses with itself to form donut-shaped cells that can stack and elongate to form tubes, or alternatively join a severed process, as in neuronal regeneration. Extracellular vesicle fusion: AFF-1 proteins can mediate the fusion between a vesicular carrier and the cell. Phagocytosis (EFF-1–mediated) and endocytosis (AFF-1–mediated): Fission events occur to seal the fission pore of the forming intracellular vesicle. Note that while endoplasmic fusogens (e.g., SNAREs and atlastins) act from the cytoplasmic space (light blue areas), EFF-1 and AFF-1 cell–cell fusogens induce fusion from the extracellular space (exoplasmic fusogens in white areas).
Similar articles
- Structural Insights into Membrane Fusion Mediated by Convergent Small Fusogens.
Yang Y, Margam NN. Yang Y, et al. Cells. 2021 Jan 15;10(1):160. doi: 10.3390/cells10010160. Cells. 2021. PMID: 33467484 Free PMC article. Review. - The hallmarks of cell-cell fusion.
Hernández JM, Podbilewicz B. Hernández JM, et al. Development. 2017 Dec 15;144(24):4481-4495. doi: 10.1242/dev.155523. Development. 2017. PMID: 29254991 Review. - Evolutionarily related small viral fusogens hijack distinct but modular actin nucleation pathways to drive cell-cell fusion.
Chan KMC, Arthur AL, Morstein J, Jin M, Bhat A, Schlesinger D, Son S, Stevens DA, Drubin DG, Fletcher DA. Chan KMC, et al. Proc Natl Acad Sci U S A. 2021 Jan 5;118(1):e2007526118. doi: 10.1073/pnas.2007526118. Proc Natl Acad Sci U S A. 2021. PMID: 33443166 Free PMC article. - Virus and cell fusion mechanisms.
Podbilewicz B. Podbilewicz B. Annu Rev Cell Dev Biol. 2014;30:111-39. doi: 10.1146/annurev-cellbio-101512-122422. Epub 2014 Jun 27. Annu Rev Cell Dev Biol. 2014. PMID: 25000995 Review. - Genetic basis of cell-cell fusion mechanisms.
Aguilar PS, Baylies MK, Fleissner A, Helming L, Inoue N, Podbilewicz B, Wang H, Wong M. Aguilar PS, et al. Trends Genet. 2013 Jul;29(7):427-37. doi: 10.1016/j.tig.2013.01.011. Epub 2013 Feb 27. Trends Genet. 2013. PMID: 23453622 Free PMC article. Review.
Cited by
- Mitochondrial transplantation: a promising strategy for treating degenerative joint diseases.
Luo H, Lai Y, Tang W, Wang G, Shen J, Liu H. Luo H, et al. J Transl Med. 2024 Oct 15;22(1):941. doi: 10.1186/s12967-024-05752-0. J Transl Med. 2024. PMID: 39407249 Free PMC article. Review. - How Much Do You Fuse? A Comparison of Cell Fusion Assays in a Breast Cancer Model.
Sieler M, Dörnen J, Dittmar T. Sieler M, et al. Int J Mol Sci. 2024 May 23;25(11):5668. doi: 10.3390/ijms25115668. Int J Mol Sci. 2024. PMID: 38891857 Free PMC article. - Role of isotropic lipid phase in the fusion of photosystem II membranes.
Böde K, Javornik U, Dlouhý O, Zsíros O, Biswas A, Domonkos I, Šket P, Karlický V, Ughy B, Lambrev PH, Špunda V, Plavec J, Garab G. Böde K, et al. Photosynth Res. 2024 Aug;161(1-2):127-140. doi: 10.1007/s11120-024-01097-3. Epub 2024 Apr 25. Photosynth Res. 2024. PMID: 38662326 Free PMC article. - No evidence for a direct extracellular interaction between human Fc receptor-like 3 (MAIA) and the sperm ligand IZUMO1.
Bianchi E, Jiménez-Movilla M, Cots-Rodríguez P, Viola C, Wright GJ. Bianchi E, et al. Sci Adv. 2024 Feb 23;10(8):eadk6352. doi: 10.1126/sciadv.adk6352. Epub 2024 Feb 21. Sci Adv. 2024. PMID: 38381819 Free PMC article. - Sperm induction of somatic cell-cell fusion as a novel functional test.
Brukman NG, Valansi C, Podbilewicz B. Brukman NG, et al. Elife. 2024 Jan 24;13:e94228. doi: 10.7554/eLife.94228. Elife. 2024. PMID: 38265078 Free PMC article.
References
- Aichel O. 1911. About cell fusion with qualitatively abnormal chromosome distribution as cause for tumor formation / Über zellverschmelzung mit qualitativ abnormer chromosomenverteilung als ursache der geschwulstbildung. In Vorträge und aufsätze über entvickelungsmechanik der organismen. Roux W., editor. Wilhelm Engelmann, Leipzig: 92–111.
- Alper S., and Podbilewicz B.. 2008. Cell Fusion in Caenorhabditis elegans. In Methods in molecular biology. Humana Press, Clifton, NJ. 53–74. - PubMed
- Antony J.M., Ellestad K.K., Hammond R., Imaizumi K., Mallet F., Warren K.G., and Power C.. 2007. The human endogenous retrovirus envelope glycoprotein, syncytin-1, regulates neuroinflammation and its receptor expression in multiple sclerosis: a role for endoplasmic reticulum chaperones in astrocytes. J. Immunol. 179:1210–1224. 10.4049/jimmunol.179.2.1210 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources