Earth history and the passerine superradiation - PubMed (original) (raw)
. 2019 Apr 16;116(16):7916-7925.
doi: 10.1073/pnas.1813206116. Epub 2019 Apr 1.
Daniel J Field 2 3, Daniel T Ksepka 4, F Keith Barker 5 6, Alexandre Aleixo 7, Michael J Andersen 8 9, Per Alström 10 11 12, Brett W Benz 13 14 15, Edward L Braun 16, Michael J Braun 17 18, Gustavo A Bravo 19 20 21, Robb T Brumfield 22 23, R Terry Chesser 24, Santiago Claramunt 25 26, Joel Cracraft 13, Andrés M Cuervo 27, Elizabeth P Derryberry 28, Travis C Glenn 29, Michael G Harvey 28, Peter A Hosner 17 30, Leo Joseph 31, Rebecca T Kimball 16, Andrew L Mack 32, Colin M Miskelly 33, A Townsend Peterson 34, Mark B Robbins 34, Frederick H Sheldon 22 23, Luís Fábio Silveira 21, Brian Tilston Smith 13, Noor D White 17 18, Robert G Moyle 34, Brant C Faircloth 1 23
Affiliations
- PMID: 30936315
- PMCID: PMC6475423
- DOI: 10.1073/pnas.1813206116
Earth history and the passerine superradiation
Carl H Oliveros et al. Proc Natl Acad Sci U S A. 2019.
Abstract
Avian diversification has been influenced by global climate change, plate tectonic movements, and mass extinction events. However, the impact of these factors on the diversification of the hyperdiverse perching birds (passerines) is unclear because family level relationships are unresolved and the timing of splitting events among lineages is uncertain. We analyzed DNA data from 4,060 nuclear loci and 137 passerine families using concatenation and coalescent approaches to infer a comprehensive phylogenetic hypothesis that clarifies relationships among all passerine families. Then, we calibrated this phylogeny using 13 fossils to examine the effects of different events in Earth history on the timing and rate of passerine diversification. Our analyses reconcile passerine diversification with the fossil and geological records; suggest that passerines originated on the Australian landmass ∼47 Ma; and show that subsequent dispersal and diversification of passerines was affected by a number of climatological and geological events, such as Oligocene glaciation and inundation of the New Zealand landmass. Although passerine diversification rates fluctuated throughout the Cenozoic, we find no link between the rate of passerine diversification and Cenozoic global temperature, and our analyses show that the increases in passerine diversification rate we observe are disconnected from the colonization of new continents. Taken together, these results suggest more complex mechanisms than temperature change or ecological opportunity have controlled macroscale patterns of passerine speciation.
Keywords: Passeriformes; biogeography; climate; diversification; macroevolution.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
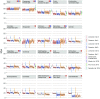
Fig. 1.
Family-level phylogenetic relationships in passerines reconciled from concatenation and coalescent analyses (connects to top of Fig. 2 at the circled star). Maximum likelihood bootstrap support (BS) values are indicated by boxes (BS > 70) or circles (BS < 70) at nodes. Node ages were estimated using 13 fossil calibrations in BEAST on nodes indicated by empty blue circles; 95% credible intervals are shown with orange bars. Ancestral areas were estimated under the DEC + j model using BioGeoBEARS from the distribution of clades represented by each tip [shown by boxes at tips coded according to the map (Inset)]. Light blue and pink bars indicate Oligocene glaciation and warming events, respectively. The estimate of Cenozoic global surface temperatures (red curve) was taken from ref. . Branches with the strongest support for diversification rate shifts are indicated by pink arrows in their descendant node for internal branches or at the base of the branch for terminal branches. Geological and climatic events are indicated above the timeline with descriptions provided in the key (Inset). Plei., Pleistocene; Plio., Pliocene.
Fig. 2.
Family-level phylogenetic relationships in passerines reconciled from concatenation and coalescent analyses (connects to bottom of Fig. 1 at the circled star). Biogeographic reconstruction including fossil taxa (Inset, tree) yields identical ancestral areas for crown lineages of passerines, suboscines, and oscines (also
SI Appendix, Fig. S8
). Plei., Pleistocene; Plio., Pliocene.
Fig. 3.
Comparison of date estimates of key nodes in passerine phylogeny across three fossil calibration schemes (sets A–C; a detailed description is provided in Materials and Methods) using log-normally distributed priors. Bars represent 95% credible intervals of joint priors [Markov chain Monte Carlo (MCMC) without data] and posteriors of random samples of 25 loci. Color boxes in the heading for each node indicate the set(s) of calibrations in which the node was used as a calibration point. Date estimates from previous studies (–5, 19) for the same node are also shown, if available.
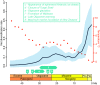
Fig. 4.
Estimate of episodic diversification rate of passerines (solid line) and 95% credible interval (light blue band) based on RevBayes analysis of our chronogram (Figs. 1 and 2) across time periods of 2 million y. Global deep ocean temperature data used in the analysis (dotted red line) were taken from ref. . Geological and climatic events from Figs. 1 and 2 (excluding events a and b) are indicated above the time line with descriptions provided in the key (Inset). Plei., Pleistocene; Plio., Pliocene.
Similar articles
- New Zealand Passerines Help Clarify the Diversification of Major Songbird Lineages during the Oligocene.
Gibb GC, England R, Hartig G, McLenachan PA, Taylor Smith BL, McComish BJ, Cooper A, Penny D. Gibb GC, et al. Genome Biol Evol. 2015 Oct 15;7(11):2983-95. doi: 10.1093/gbe/evv196. Genome Biol Evol. 2015. PMID: 26475316 Free PMC article. - Going to extremes: contrasting rates of diversification in a recent radiation of new world passerine birds.
Barker FK, Burns KJ, Klicka J, Lanyon SM, Lovette IJ. Barker FK, et al. Syst Biol. 2013 Mar;62(2):298-320. doi: 10.1093/sysbio/sys094. Epub 2012 Dec 9. Syst Biol. 2013. PMID: 23229025 - Dating the diversification of the major lineages of Passeriformes (Aves).
Ericson PG, Klopfstein S, Irestedt M, Nguyen JM, Nylander JA. Ericson PG, et al. BMC Evol Biol. 2014 Jan 15;14:8. doi: 10.1186/1471-2148-14-8. BMC Evol Biol. 2014. PMID: 24422673 Free PMC article. - The Paleogene fossil record of birds in Europe.
Mayr G. Mayr G. Biol Rev Camb Philos Soc. 2005 Nov;80(4):515-42. doi: 10.1017/S1464793105006779. Biol Rev Camb Philos Soc. 2005. PMID: 16221327 Review. - Bird evolution in the Eocene: climate change in Europe and a Danish fossil fauna.
Lindow BE, Dyke GJ. Lindow BE, et al. Biol Rev Camb Philos Soc. 2006 Nov;81(4):483-99. doi: 10.1017/S146479310600707X. Epub 2006 Aug 8. Biol Rev Camb Philos Soc. 2006. PMID: 16893476 Review.
Cited by
- Macroevolutionary drivers of morphological disparity in the avian quadrate.
Kuo PC, Navalón G, Benson RBJ, Field DJ. Kuo PC, et al. Proc Biol Sci. 2024 Feb 28;291(2017):20232250. doi: 10.1098/rspb.2023.2250. Epub 2024 Feb 21. Proc Biol Sci. 2024. PMID: 38378144 Free PMC article. - Direct quantification of skeletal pneumaticity illuminates ecological drivers of a key avian trait.
Burton MGP, Benson RBJ, Field DJ. Burton MGP, et al. Proc Biol Sci. 2023 Mar 29;290(1995):20230160. doi: 10.1098/rspb.2023.0160. Epub 2023 Mar 15. Proc Biol Sci. 2023. PMID: 36919426 Free PMC article. - Divergence time estimation of Galliformes based on the best gene shopping scheme of ultraconserved elements.
Chen D, Hosner PA, Dittmann DL, O'Neill JP, Birks SM, Braun EL, Kimball RT. Chen D, et al. BMC Ecol Evol. 2021 Nov 22;21(1):209. doi: 10.1186/s12862-021-01935-1. BMC Ecol Evol. 2021. PMID: 34809586 Free PMC article. - Convergent evolution in dippers (Aves, Cinclidae): The only wing-propelled diving songbirds.
Smith NA, Koeller KL, Clarke JA, Ksepka DT, Mitchell JS, Nabavizadeh A, Ridgley RC, Witmer LM. Smith NA, et al. Anat Rec (Hoboken). 2022 Jul;305(7):1563-1591. doi: 10.1002/ar.24820. Epub 2021 Nov 23. Anat Rec (Hoboken). 2022. PMID: 34813153 Free PMC article. - Whole-genome analysis across 10 songbird families within Sylvioidea reveals a novel autosome-sex chromosome fusion.
Sigeman H, Ponnikas S, Hansson B. Sigeman H, et al. Biol Lett. 2020 Apr;16(4):20200082. doi: 10.1098/rsbl.2020.0082. Epub 2020 Apr 22. Biol Lett. 2020. PMID: 32315592 Free PMC article.
References
- Feduccia A. Explosive evolution in tertiary birds and mammals. Science. 1995;267:637–638. - PubMed
- Prum RO, et al. A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature. 2015;526:569–573. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources