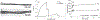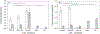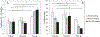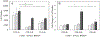Engineering small-caliber vascular grafts from collagen filaments and nanofibers with comparable mechanical properties to native vessels - PubMed (original) (raw)
Engineering small-caliber vascular grafts from collagen filaments and nanofibers with comparable mechanical properties to native vessels
Fan Zhang et al. Biofabrication. 2019.
Abstract
At the present time, there is no successful synthetic, off-the-shelf small-caliber vascular graft (<6 mm) for the repair or bypass of the coronary or carotid arteries. This stimulates on-going investigations to fabricate an artificial vascular graft that has both sufficient mechanical properties as well as superior biological performance. Collagen has long been considered as a viable material to encourage cell recruitment, tissue regeneration, and revascularization, but its use has been limited by its inferior mechanical properties. In this study, novel electrochemically aligned collagen filaments were used to engineer a bilayer small-caliber vascular graft, by circular knitting the collagen filaments and electrospinning collagen nanofibers. The collagen prototype grafts showed significantly greater bursting strength under dry and hydrated conditions to that of autografts such as the human internal mammary artery and the saphenous vein (SV). The suture retention strength was sufficient under dry condition, but that under hydrated condition needs to be further improved. The radial dynamic compliance of the collagen grafts was similar to that of the human SV. During in vitro cell culture assays with human umbilical vein endothelial cells, the prototype collagen grafts also encouraged cell adhesion and promoted cell proliferation compared to the synthetic poly(lactic acid) grafts. In conclusion, this study demonstrated the feasibility of the use of novel collagen filaments for fabricating small caliber tissue-engineered vascular grafts that provide both sufficient mechanical properties and superior biological performance.
Figures
Figure 1.
The collagen filaments were directly knitted into a tubular structure (B) on the lamb circular weft knitting machine (A).
Figure 2.
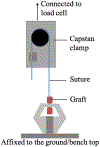
Diagram of the suture retention test set-up.
Figure 3.
Characterization of the 2-ply collagen filaments under dry (A) and hydrated (B) conditions. (C) Representative loadextension curves of dry and hydrated collagen filaments. (D) Swelling ratio of the uncrosslinked and crosslinked collagen filaments over 24 h.
Figure 4.
Dimensions of the collagen prototype grafts. (a) The single-layer knitted collagen graft (COL-K); (b) the bilayer collagen graft with the electrospun layer on the outer surface of the knitted layer; (c) the bilayer knitted and electrospun collagen graft (COL-KE). (A) Wall thickness of the prototype grafts; (B) Outer and inner diameter of the grafts; (C) pore size of the grafts.
Figure 5.
Microscopic images of COL-K (a) and (b), COL-KE (c) and (d), and PLA-K (e) and (f) (scale bar: a, c, e = 500 _μ_m; b, f = 200 _μ_m; d = 10 _μ_m).
Figure 6.
(A) Bursting strength and (B) suture retention strength of the prototype grafts compared with the hydrated human internal mammary artery (IMA) and the human saphenous vein (SV) and the PLA-K reference graft. The data of the reference autologous grafts were obtained from [30]. * indicates statistical significant difference, p < 0.05, n = 3.
Figure 7.
(A) Radial dynamic compliance and (B) β stiffness index of the prototype grafts. * indicates statistical significant difference, p < 0.05, n = 3.
Figure 8.
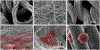
Representative microscopic images of the collagen and PLA substrates and HUVECs attached at Day 3. (a) COL-K shares a similar structure with (c) PLA-K, but PLA-K has a smoother surface and larger surface area compared to COL-K due to the multifilament feature; (b) COL-KE, on the other hand, had a different morphology. A larger number of well-attached HUVECs grew on the collagen substrate (d), (e) compared to the PLA scaffold (f). Cells have been highlighted in red.
Figure 9.
Cell attachment and proliferation determined by alamarBlue® assay over 9 days. *Indicates significant difference, p < 0.05, n = 3.
Similar articles
- A hybrid vascular graft harnessing the superior mechanical properties of synthetic fibers and the biological performance of collagen filaments.
Zhang F, Bambharoliya T, Xie Y, Liu L, Celik H, Wang L, Akkus O, King MW. Zhang F, et al. Mater Sci Eng C Mater Biol Appl. 2021 Jan;118:111418. doi: 10.1016/j.msec.2020.111418. Epub 2020 Aug 22. Mater Sci Eng C Mater Biol Appl. 2021. PMID: 33255019 - A novel polymeric fibrous microstructured biodegradable small-caliber tubular scaffold for cardiovascular tissue engineering.
Dimopoulos A, Markatos DN, Mitropoulou A, Panagiotopoulos I, Koletsis E, Mavrilas D. Dimopoulos A, et al. J Mater Sci Mater Med. 2021 Mar 1;32(2):21. doi: 10.1007/s10856-021-06490-1. J Mater Sci Mater Med. 2021. PMID: 33649939 Free PMC article. - Tissue-engineered small-caliber vascular graft based on a novel biodegradable composite fibrin-polylactide scaffold.
Tschoeke B, Flanagan TC, Koch S, Harwoko MS, Deichmann T, Ellå V, Sachweh JS, Kellomåki M, Gries T, Schmitz-Rode T, Jockenhoevel S. Tschoeke B, et al. Tissue Eng Part A. 2009 Aug;15(8):1909-18. doi: 10.1089/ten.tea.2008.0499. Tissue Eng Part A. 2009. PMID: 19125650 - Electrospun scaffolds for tissue engineering of vascular grafts.
Hasan A, Memic A, Annabi N, Hossain M, Paul A, Dokmeci MR, Dehghani F, Khademhosseini A. Hasan A, et al. Acta Biomater. 2014 Jan;10(1):11-25. doi: 10.1016/j.actbio.2013.08.022. Epub 2013 Aug 22. Acta Biomater. 2014. PMID: 23973391 Free PMC article. Review. - Tissue engineered small-diameter vascular grafts.
Schmedlen RH, Elbjeirami WM, Gobin AS, West JL. Schmedlen RH, et al. Clin Plast Surg. 2003 Oct;30(4):507-17. doi: 10.1016/s0094-1298(03)00069-5. Clin Plast Surg. 2003. PMID: 14621299 Review.
Cited by
- Computational and Experimental Characterization of Aligned Collagen across Varied Crosslinking Degrees.
Lin S, Patrawalla NY, Zhai Y, Dong P, Kishore V, Gu L. Lin S, et al. Micromachines (Basel). 2024 Jun 29;15(7):851. doi: 10.3390/mi15070851. Micromachines (Basel). 2024. PMID: 39064362 Free PMC article. - Current Strategies for Engineered Vascular Grafts and Vascularized Tissue Engineering.
Chen J, Zhang D, Wu LP, Zhao M. Chen J, et al. Polymers (Basel). 2023 Apr 24;15(9):2015. doi: 10.3390/polym15092015. Polymers (Basel). 2023. PMID: 37177162 Free PMC article. Review. - A collagen/PLA hybrid scaffold supports tendon-derived cell growth for tendon repair and regeneration.
Xie Y, Zhang F, Akkus O, King MW. Xie Y, et al. J Biomed Mater Res B Appl Biomater. 2022 Dec;110(12):2624-2635. doi: 10.1002/jbm.b.35116. Epub 2022 Jul 2. J Biomed Mater Res B Appl Biomater. 2022. PMID: 35779243 Free PMC article. - Natural and Synthetic Polymeric Biomaterials for Application in Wound Management.
Prete S, Dattilo M, Patitucci F, Pezzi G, Parisi OI, Puoci F. Prete S, et al. J Funct Biomater. 2023 Sep 3;14(9):455. doi: 10.3390/jfb14090455. J Funct Biomater. 2023. PMID: 37754869 Free PMC article. Review. - Challenges and strategies for in situ endothelialization and long-term lumen patency of vascular grafts.
Zhuang Y, Zhang C, Cheng M, Huang J, Liu Q, Yuan G, Lin K, Yu H. Zhuang Y, et al. Bioact Mater. 2020 Dec 5;6(6):1791-1809. doi: 10.1016/j.bioactmat.2020.11.028. eCollection 2021 Jun. Bioact Mater. 2020. PMID: 33336112 Free PMC article. Review.
References
- WHO 2018. Cardiovascular Dis. (https://who.int/newsroom/fact-sheets/detail/cardiovascular-diseases-(cvds))
- Ahmed M, George H and Seifalian AM 2014. The performance of a small-caliber graft for vascular reconstructions in a senescent sheep model Biomaterials 35 9033–40 - PubMed
- Yahagi K. et al. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat. Rev. Cardiol. 2016;13:79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources