Inflammatory cytokines associated with cancer growth induce mitochondria and cytoskeleton alterations in cardiomyocytes - PubMed (original) (raw)
. 2019 Nov;234(11):20453-20468.
doi: 10.1002/jcp.28647. Epub 2019 Apr 14.
Sonia Maccari 2, Barbara Ascione 3, Lucrezia Gambardella 3, Matteo Marconi 3, Massimo Spada 4, Daniele Macchia 4, Tonino Stati 2, Mario Patrizio 2, Walter Malorni 3 5, Paola Matarrese 3, Giuseppe Marano 2, Lucia Gabriele 6
Affiliations
- PMID: 30982981
- PMCID: PMC6767566
- DOI: 10.1002/jcp.28647
Inflammatory cytokines associated with cancer growth induce mitochondria and cytoskeleton alterations in cardiomyocytes
Maria Buoncervello et al. J Cell Physiol. 2019 Nov.
Abstract
Cardiac dysfunction is often observed in patients with cancer also representing a serious problem limiting chemotherapeutic intervention and even patient survival. In view of the recently established role of the immune system in the control of cancer growth, the present work has been undertaken to investigate the effects of a panel of the most important inflammatory cytokines on the integrity and function of mitochondria, as well as of the cytoskeleton, two key elements in the functioning of cardiomyocytes. Either mitochondria features or actomyosin cytoskeleton organization of in vitro-cultured cardiomyocytes treated with different inflammatory cytokines were analyzed. In addition, to investigate the interplay between tumor growth and cardiac function in an in vivo system, immunocompetent female mice were inoculated with cancer cells and treated with the chemotherapeutic drug doxorubicin at a dosing schedule able to suppress tumor growth without inducing cardiac alterations. Analyses carried out in cardiomyocytes treated with the inflammatory cytokines, such as tumor necrosis factor α (TNF-α), interferon γ (IFN-γ), interleukin 6 (IL-6), IL-8, and IL-1β revealed severe phenotypic changes, for example, of contractile cytoskeletal elements, mitochondrial membrane potential, mitochondrial reactive oxygen species production and mitochondria network organization. Accordingly, in immunocompetent mice, the tumor growth was accompanied by increased levels of the inflammatory cytokines TNF-α, IFN-γ, IL-6, and IL-8, either in serum or in the heart tissue, together with a significant reduction of ventricular systolic function. The alterations of mitochondria and of microfilament system of cardiomyocytes, due to the systemic inflammation associated with cancer growth, could be responsible for remote cardiac injury and impairment of systolic function observed in vivo.
Keywords: cardiac function; cardiomyocytes; cytoskeleton; doxorubicin; inflammatory cytokines; mitochondria; sex; tumor growth.
© 2019 The Authors. Journal of Cellular Physiology Published by Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures
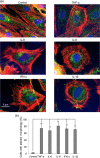
Figure 1
Inflammatory cytokines promote cytoskeleton rearrangement in in vitro‐cultured cardiomyocytes. (a) Representative micrographs obtained by IVM after TRITC‐phalloidin (red)/myosin (green)/Hoechst (blue) triple staining of H2c9 cardiomyocytes untreated (control) or treated for 48 hr with 50 ng/ml IFN‐γ, TNF‐α, IL‐6, IL‐8 or IL‐1β. (b) Bar graph shows morphometric analysis results. In ordinate the percentage of the cells with an altered shape in comparison to control cells. **p < 0.01. IFN‐γ: interferon γ; IL‐6: interleukin 6; IVM: in vitro maturation; TNF‐α: tumor necrosis factor α; TRITC: tetramethylrhodamine [Color figure can be viewed at wileyonlinelibrary.com]
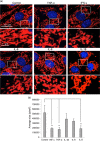
Figure 2
Inflammatory cytokines induce mitochondrial network alterations in in vitro‐cultured cardiomyocytes. (a) Representative micrographs obtained by IVM showing the mitochondrial network of H2c9 cardiomyocytes untreated (control) or treated for 48 hr with 50 ng/ml IFN‐γ, TNF‐α, IL‐6, IL‐8, or IL‐1β after staining with anti‐TOM20 (red) and counterstaining with Hoechst (blue). In the bottom pictures, magnification of the boxed areas. (b) Bar graph showing morphometric analysis performed by using the ImageJ software. In ordinate average mitochondrial area is expressed as pixel2. Data are reported as mean value ± SD of the results obtained by analyzing at least 30 cells for each sample. IFN‐γ: interferon γ; IL‐6: interleukin 6; IVM: in vitro maturation; SD: standard deviation; TNF‐α: tumor necrosis factor α [Color figure can be viewed at wileyonlinelibrary.com]
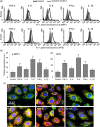
Figure 3
Inflammatory cytokines induce upregulation of phospo‐DRP1 and hFIS in in vitro‐cultured cardiomyocytes. (a) Representative histograms of the cytofluorimetric analysis of expression level of p‐DRP1 (first row) and hFIS (second row). (b) Bar graphs showings the mean ± SD of the results obtained from three independent experiments expressed as median fluorescence intensity. **p < 0.01. (c) Representative micrographs obtained by IVM showing mitochondrial network and hFIS of H2c9 cardiomyocytes untreated (control) or treated for 48 hr with 50 ng/ml IFN‐γ, TNF‐α, IL‐6, IL‐8, or IL‐1β after staining with anti‐mitochondria (green), anti‐hFIS (red), and counterstaining with Hoechst (blue). Note yellow fluorescence in cells treated with cytokines that indicate mitochondrial localization of hFIS. DRP1: dynamin‐related protein 1; IFN‐γ: interferon γ; IL‐6: interleukin 6; IVM: in vitro maturation; SD: standard deviation; TNF‐α: tumor necrosis factor α [Color figure can be viewed at wileyonlinelibrary.com]
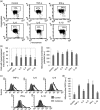
Figure 4
Inflammatory cytokines induce an increase of the mitochondrial membrane potential and mitochondrial ROS production in in vitro‐cultured cardiomyocytes. (a) Representative dot plots of the cytofluorimetric analysis of MMP, performed by using JC‐1. Numbers in the boxed areas indicate the percentage of cells with high MMP. (b) Left panel. Bar graph showing the results of the analysis of MMP obtained by using JC‐1 from three independent experiments and reported as the percentage ± SD of cells with hyperpolarized mitochondria. Right panel. Bar graphs showings the mean ± SD of the results obtained by using TMRM from three independent experiments expressed as median fluorescence intensity. (c) Representative histograms of the cytofluorimetric analysis of mitochondrial ROS, performed by using the MitoSox probe. (d) Bar graph shows the mean ± SD of the results obtained from three independent experiments expressed as median fluorescence intensity. **p < 0.01. IFN‐γ: interferon γ; IL‐6: interleukin 6; MMP: mitochondrial membrane potential; ROS: reactive oxygen species; SD: standard deviation; TMRM: tetramethylrhodamine; TNF‐α: tumor necrosis factor α
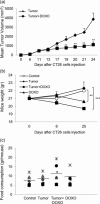
Figure 5
Effect of DOXO on tumor burden in the CT26 tumor‐bearing mice. (a) BALB/c mice have injected sc with 2 × 106 CT26 cells and tumor size was measured over time. After 6 and 13 days, mice have treated iv with DOXO (10 mg/kg). Data represent the mean tumor volume ± SD. One representative experiment out of three is shown. **p ≤ 0.01 versus untreated mice bearing the CT26 tumor. (b) The body weight of mice was expressed as total weight minus tumor weight and measured before tumor cells inoculation (Day 0), before DOXO treatment when tumors were palpable (Day 6) and at the end of the study (Day 25). (c) Effect of DOXO on cumulative food intake in mice. Food consumption was monitored every 3 days and the mean of total measures is shown. DOXO: doxorubicin; s.c.: subcutaneously; SD: standard deviation; i.v.: intravenously
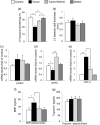
Figure 6
CRC tumor growth induces very early cardiac dysfunction. (a) CT26‐bearing mice display a reduced FS when compared with the untreated control. DOXO administration prevented the development of cardiac dysfunction. Also, doxorubicin alone did not affect cardiac function. (b) Neither cancer growth nor DOXO altered LV diastolic wall thickness, an echocardiographic marker of cardiac remodeling. (c) mRNA levels of β‐MHC (d) NPPA, and (e) TNF‐α were assessed from heart tissue samples by q‐PCR after being normalized by the mRNA level of GAPDH. Untreated heart tissue samples from BALB/c mice (control) were used as negative control. Quantitative evaluation of (f) BNP and (g) troponin I in animal plasma was performed by ELISA assay and expressed as pg/ml. Data reported are the mean ± SD of the results obtained by analyzing at least six animals in three independent experiments; each experimental analysis was performed in triplicate. *p < 0.05, **p < 0.01. BNP: B‐type natriuretic peptide; CRC: colorectal cancer; DOXO: doxorubicin; ELISA: enzyme‐linked immunosorbent assay; FS: fractional shortening; LV: left ventricular; mRNA: messenger RNA; NPPA: natriuretic peptide A ; q‐PCR: quantitative polymerase chain reaction; SD: standard deviation; TNF‐α: tumor necrosis factor α
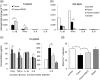
Figure 7
Tumor‐induced heart damage correlates with systemic immune activation. Effects of DOXO on tissue and systemic cytokines. RNA was purified from tumors (a) and spleen (b) and qRT‐PCR for the indicated cytokines was carried out. Histograms represent the absolute mRNA amount normalized to β‐actin in samples run in triplicate (mean ± SD). One representative experiment of three is shown. (c) Quantitative ELISA of IFN‐γ, TNF‐α, IL‐1β, IL‐6, and IL‐8 in plasma of healthy mice untreated or treated with DOXO and in mice CT26‐bearing tumor, untreated or treated with DOXO. Data are the mean ± SD among six animals for each experimental point performed in triplicate. (d) TAC was assayed by using a specific colorimetric test, which measures the total antioxidant power in terms of the sample's ability to reduce copper. Each value reported represents the mean ± SD of results obtained by analyzing at least six animals for each experimental point performed in triplicate. *p < 0.05, **p < 0.01. DOXO: doxorubicin; ELISA: enzyme‐linked immunosorbent assay; IFN‐γ: interferon γ; IL‐6: interleukin 6; mRNA: messenger RNA; qRT‐PCR: quantitative real‐time polymerase chain reaction; SD: standard deviation; TAC: total antioxidant capacity
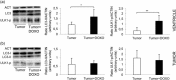
Figure 8
Involvement of autophagy in tumor‐induced heart damage. Western blot analysis using anti‐LC3 and p‐ULK (Ser 777) antibodies (a) in ventricle tissue or (b) in explanted tumors from CT26‐bearing mice either untreated (tumor) or treated with DOXO (tumor + DOXO). Loading control was evaluated using anti‐actin MAb. Two animals representative among six are shown. Bar graphs show densitometric analysis. *p < 0.05, **p < 0.01. DOXO: doxorubicin; MAb: monoclonal antibody
Similar articles
- IL-1beta and IL-6 act synergistically with TNF-alpha to alter cardiac contractile function after burn trauma.
Maass DL, White J, Horton JW. Maass DL, et al. Shock. 2002 Oct;18(4):360-6. doi: 10.1097/00024382-200210000-00012. Shock. 2002. PMID: 12392281 - Co-Exposure of Cardiomyocytes to IFN-γ and TNF-α Induces Mitochondrial Dysfunction and Nitro-Oxidative Stress: Implications for the Pathogenesis of Chronic Chagas Disease Cardiomyopathy.
Nunes JPS, Andrieux P, Brochet P, Almeida RR, Kitano E, Honda AK, Iwai LK, Andrade-Silva D, Goudenège D, Alcântara Silva KD, Vieira RS, Levy D, Bydlowski SP, Gallardo F, Torres M, Bocchi EA, Mano M, Santos RHB, Bacal F, Pomerantzeff P, Laurindo FRM, Teixeira PC, Nakaya HI, Kalil J, Procaccio V, Chevillard C, Cunha-Neto E. Nunes JPS, et al. Front Immunol. 2021 Nov 11;12:755862. doi: 10.3389/fimmu.2021.755862. eCollection 2021. Front Immunol. 2021. PMID: 34867992 Free PMC article. - Influence of pro-inflammatory (IL-1 alpha, IL-6, TNF-alpha, IFN-gamma) and anti-inflammatory (IL-4) cytokines on chondrocyte function.
Schuerwegh AJ, Dombrecht EJ, Stevens WJ, Van Offel JF, Bridts CH, De Clerck LS. Schuerwegh AJ, et al. Osteoarthritis Cartilage. 2003 Sep;11(9):681-7. doi: 10.1016/s1063-4584(03)00156-0. Osteoarthritis Cartilage. 2003. PMID: 12954239 - Could interferon-gamma be a therapeutic target for treating heart failure?
Levick SP, Goldspink PH. Levick SP, et al. Heart Fail Rev. 2014 Mar;19(2):227-36. doi: 10.1007/s10741-013-9393-8. Heart Fail Rev. 2014. PMID: 23589353 Free PMC article. Review. - Cancer-A Major Cardiac Comorbidity With Implications on Cardiovascular Metabolism.
Finke D, Heckmann MB, Frey N, Lehmann LH. Finke D, et al. Front Physiol. 2021 Nov 26;12:729713. doi: 10.3389/fphys.2021.729713. eCollection 2021. Front Physiol. 2021. PMID: 34899373 Free PMC article. Review.
Cited by
- An Immunocompetent Microphysiological System to Simultaneously Investigate Effects of Anti-Tumor Natural Killer Cells on Tumor and Cardiac Microtissues.
Nguyen OTP, Misun PM, Lohasz C, Lee J, Wang W, Schroeder T, Hierlemann A. Nguyen OTP, et al. Front Immunol. 2021 Dec 2;12:781337. doi: 10.3389/fimmu.2021.781337. eCollection 2021. Front Immunol. 2021. PMID: 34925361 Free PMC article. - Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome.
Paul BD, Lemle MD, Komaroff AL, Snyder SH. Paul BD, et al. Proc Natl Acad Sci U S A. 2021 Aug 24;118(34):e2024358118. doi: 10.1073/pnas.2024358118. Proc Natl Acad Sci U S A. 2021. PMID: 34400495 Free PMC article. Review. - Mitochondrial fission enhances IL-6-induced metastatic potential in ovarian cancer via ERK1/2 activation.
Lee J, Han Y, Kim S, Jo H, Wang W, Cho U, Kim SI, Kim B, Song YS. Lee J, et al. Cancer Sci. 2024 May;115(5):1536-1550. doi: 10.1111/cas.16064. Epub 2024 Mar 3. Cancer Sci. 2024. PMID: 38433313 Free PMC article. - Doxorubicin Paradoxically Ameliorates Tumor-Induced Inflammation in Young Mice.
Abdelgawad IY, Grant MKO, Popescu FE, Largaespada DA, Zordoky BN. Abdelgawad IY, et al. Int J Mol Sci. 2021 Aug 21;22(16):9023. doi: 10.3390/ijms22169023. Int J Mol Sci. 2021. PMID: 34445729 Free PMC article. - More than a key-the pathological roles of SARS-CoV-2 spike protein in COVID-19 related cardiac injury.
Lin Z. Lin Z. Sports Med Health Sci. 2023 Mar 30;6(3):209-20. doi: 10.1016/j.smhs.2023.03.004. Online ahead of print. Sports Med Health Sci. 2023. PMID: 37361919 Free PMC article. Review.
References
- Abdullah, C. S. , Alam, S. , Aishwarya, R. , Miriyala, S. , Bhuiyan, M. A. N. , Panchatcharam, M. , … Bhuiyan, M. S. (2019). Doxorubicin‐induced cardiomyopathy associated with inhibition of autophagic degradation process and defects in mitochondrial respiration. Scientific Report, 9, 2002 10.1038/s41598-018-37862-3 - DOI - PMC - PubMed
- Arámbula‐Garza, E. , Castillo‐Martínez, L. , González‐Islas, D. , Orea‐Tejeda, A. , Santellano‐Juárez, B. , Keirns‐Davies, C. , … Pablo‐Santiago, R. (2016). Association of cardiac cachexia and atrial fibrillation in heart failure patients. International Journal of Cardiology, 223, 863–866. 10.1016/j.ijcard.2016.08.318 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources