Sestrin modulator NV-5138 produces rapid antidepressant effects via direct mTORC1 activation - PubMed (original) (raw)
Sestrin modulator NV-5138 produces rapid antidepressant effects via direct mTORC1 activation
Taro Kato et al. J Clin Invest. 2019.
Abstract
Preclinical studies demonstrate that rapid acting antidepressants, including ketamine require stimulation of mTORC1 signaling. This pathway is regulated by neuronal activity, endocrine and metabolic signals, notably the amino acid leucine, which activates mTORC1 signaling via binding to the upstream regulator sestrin. Here, we examined the antidepressant actions of NV-5138, a novel highly selective small molecule modulator of sestrin that penetrates the blood brain barrier. The results demonstrate that a single dose of NV-5138 produced rapid and long-lasting antidepressant effects, and rapidly reversed anhedonia caused by chronic stress exposure. The antidepressant actions of NV-5138 required BDNF release as the behavioral responses are blocked by infusion of a BDNF neutralizing antibody into the medial prefrontal cortex (mPFC) or in mice with a knock-in of a BDNF polymorphism that blocks activity dependent BDNF release. NV-5138 administration also rapidly increased synapse number and function in the mPFC, and reversed the synaptic deficits caused by chronic stress. Together, the results demonstrate that NV-5138 produced rapid synaptic and antidepressant behavioral responses via activation of the mTORC1 pathway and BDNF signaling, indicating that pharmacological modulation of sestrin is a novel approach for development of rapid acting antidepressants.
Keywords: Depression; Neuroscience.
Conflict of interest statement
Conflict of interest: This work was funded by Navitor Pharmaceuticals. RSD has consulted and/or received research support from Naurex, Allergan, Lilly, Forest, Johnson & Johnson, Taisho Pharmaceutical Co., Sunovion, and Navitor.
Figures
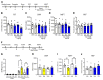
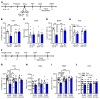
Figure 1. NV-5138 shows ketamine-like antidepressant actions in rodent models of depression.
(A) Beginning 24 hours after NV-5138 (40, 80, or 160 mg/kg p.o.) administration, behavioral studies were conducted over 3 consecutive days (B–E). NV-5138 treatment significantly decreased immobility time and latency to feed at 160 mg/kg in (B) the FST (F4,35 = 3.24, P < 0.01) and (D**) the NSFT (F4,35 = 2.67, P < 0.05), respectively. No significant effects were seen in (**C**) locomotor activity (F4,35 = 0.536, _P_ > 0.05) or (E) HCF (F4,35 = 0.223, P > 0.05). (F) Beginning 24 hours after ketamine (10 mg/kg i.p.) or NV-5138 (160 mg/kg, p.o.) administration, behavioral studies were conducted over the next 3 days (G–J**). Both ketamine and NV-5138 significantly increased female urine sniffing time and decreased latency to feed in (G) the FUST and (I) the NSFT, respectively. No significant effects were seen in (H) locomotor activity or (J) HCF. The results are shown as mean ± SEM. n = 8/group. *P < 0.05; **P < 0.01, Tukey’s multiple comparison test, following significant results of 1-way ANOVA (B–E, H–J) or Student’s t test (G). Veh, vehicle; Leu, leucine; Ket, ketamine; NV, NV-5138; LMA, locomotor activity test.
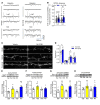
Figure 2. Single-dose NV-5183 produces long-lasting antidepressant effects, and repeated low-dose NV-5138 also produces antidepressant effects.
(A) Three or seven days after NV-5138 (160 mg/kg) or ketamine (10 mg/kg) administration, the FST was conducted. Three days after the FST, the NSFT was conducted. Both NV-5138 and ketamine significantly decreased immobility time in the FST 3 and 7 days after administration (B and D) (NV-5138; F2,21 = 5.82, P < 0.01, ketamine; F2,21 = 5.47, _P_ < 0.05) but had no effect on latency to feed in the NSFT on day 10 (C** and E). (F) Low-dose NV-5138 was administered once a day for 7 days, and ketamine was administered on alternate days over the same time frame. Twenty-four hours after the last administration, behavioral studies were conducted over the next 3 days (G–J). Both ketamine and 80 mg/kg of NV-5138 significantly decreased immobility time in the FST (I) (F3,28 = 4.05, P < 0.05) and latency to feed in the NSFT (**K**) (F3,28 = 7.29, _P_ < 0.001). No significant effects were seen in (**H**) locomotor activity (F3,27 = 0.500, _P_ > 0.05) or (J) HCF (F3,28 = 0.380, P > 0.05). The results are shown as mean ± SEM. n = 8/group. *P < 0.05; **P < 0.01, Tukey’s multiple comparison test, following significant results of 1-way ANOVA (B, D, G–J**) or Student’s t test (C and E).
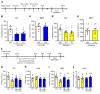
Figure 3. NV-5138 reverses the behavioral and synaptic deficits caused by CUS.
(A) Schematic for CUS experiment and effect of NV-5138 (160 mg/kg, day 20 of CUS) on the SPT and NSFT (on days 21 and 22 of CUS). CUS was continued throughout the behavioral testing and tissue sampling on day 26. After a short washout period, a second dose of NV-5138 was administered on CUS day 25 and tissue was collected on day 26. (B–D) Results are the mean ± SEM. n = 13–14/group. (B) CUS decreased sucrose preference and NV-5138 reversed this effect; there was a significant interaction between CUS and NV-5138 administration (F1,50 = 5.20, P < 0.05, effect of CUS: F1,50 = 2.21, _P_ > 0.05, effect of NV-5138: F1,50 = 3.27, P > 0.05). Also, a 3-way ANOVA analysis revealed a significant interaction among CUS, NV-5138 administration, and sucrose consumption (F1,50 = 4.02, P = 0.05). (C) CUS increased the latency to feed in NSFT, and NV-5138 reversed this effect (F1,50 = 1.89, P > 0.05, effect of CUS: F1,50 = 17.52, P < 0.001, effect of NV-5138: F1,50 = 43.4, _P_ < 0.0001). (D**) There was no significant effect on HCF (F1,50 = 0.0679, P > 0.05. (E) CUS significantly decreased body weight (F1,104 = 47.06, P < 0.0001, effect of day: F1,104 = 2612, P < 0.0001, effect of CUS: F1,50 = 49.86, P < 0.0001). Results are shown as mean ± SEM. n = 13–14/group. *P < 0.05; **P < 0.01, 2-way ANOVA and Tukey’s multiple comparison test. (F and G) CUS decreased levels of the postsynaptic proteins GluR1 and PSD95 in PFC, and NV-5138 reversed these deficits (GluR1, F2,19 = 7.95, P < 0.01; PSD95, F2,19 = 6.31, P < 0.01). Results are shown as mean ± SEM. n = 7–8/group. *P < 0.05; **P < 0.01, ****P < 0.001, 1-way ANOVA (B–D**, F, G) and Student’s t test (E). NS, NSFT; V, vehicle.
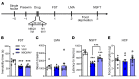
Figure 4. Antidepressant actions of NV-5138 are dependent on activation of mTORC1 signaling.
(A) Rats were administered vehicle or NV-5138 (160 mg/kg) and PFC dissections were collected 1 hour later. (B) Diagram showing postsynaptic signaling. Levels of the phosphorylated and activated forms of (C) mTOR, (D) p70S6K, and (E) 4EBP1 as determined by Western blot analysis were increased by NV-5138 and ketamine; levels of total proteins or GAPDH were also measured to control for loading differences. Results are shown as mean ± SEM. n = 6/group. *P < 0.05; **_P_ < 0.01, Student’s _t_ test. (**F**) Rats were implanted with bilateral cannula in the mPFC and allowed to recover for approximately 2 weeks. (**G**) The mTORC1 inhibitor rapamycin was infused into the mPFC 30 minutes prior to administration of vehicle or NV-5138. Twenty-four hours after NV-5138 administration, behavioral studies were initiated and conducted over the next 3 days (**H**–**K**). NV-5138 treatment significantly decreased immobility time and latency to feed, but these effects were blocked by rapamycin in (**H**) the FST (F2,17 = 20.46, _P_ < 0.001) and (**J**) the NSFT (F2,17 = 5.36, _P_ < 0.05), respectively. No significant effects were seen in (**I**) LMA (F2,17 = 0.200, _P_ > 0.05) or (K) HCF (F2,17 = 0.814, P > 0.05). Results are shown as mean ± SEM. n = 6–7. *P < 0.05; **P < 0.01, Tukey’s multiple comparisons test, following significant results of 1-way ANOVA. Rap, rapamycin.
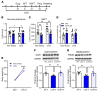
Figure 5. BDNF is required for antidepressant actions of NV-5138.
(A–E) The antidepressant actions of NV-5138 are blocked by infusion of BDNF nAb into the mPFC. (A) BDNF nAb or control IgG (0.5 μg/side) was infused into the mPFC 30 minutes prior to administration of vehicle or NV-5138 (160 mg/kg, p.o.). (B and D) NV-5138 significantly decreased immobility time (B) in the FST (effect of NV-5138: F1,29 = 14.6, P < 0.001; mPFC infusion: F1,29 = 13.4, _P_ < 0.001; interaction: F1,29 = 14.9, _P_ < 0.001) and latency to feed (D**) in the NSFT (effect of NV-5138: F1,29 = 39.4, P < 0.001; mPFC infusion: F1,29 = 11.2, _P_ = 0.002; interaction: F1,29 = 18.0, _P_ < 0.001), and these effects were blocked by infusion of the BDNF nAb. (**C** and **E**) There were no significant effects on locomotor activity (effect of NV-5138: F1,30 = 0.01, _P_ = 0.92; mPFC infusion: F1,30 = 0.07, _P_ = 0.79; interaction: F1,30 = 0.03, _P_ = 0.86) or HCF (effect of NV-5138: F1,30 = 0.11, _P_ = 0.74; mPFC infusion: F1,30 = 2.93, _P_ = 0.1; interaction: F1,30 = 1.23, _P_ = 0.28). (**F**–**J**) The antidepressant actions of NV-5138 are attenuated in BDNF Val66Met knockin mice. (**F**) Experimental time line for behavioral testing of animals after vehicle or NV-5138 administration. In the BDNF Val/Val mice, NV-5138 treatment significantly decreased immobility time (**G**) in the FST (effect of NV-5138: F1,64 = 12.7, _P_ < 0.001; genotype: F2,64 = 1.88, _P_ = 0.16; interaction: F2,64 = 3.98, _P_ = 0.02) and latency to feed (**I**) in the NSFT (effect of NV-5138: F1,65 = 13, _P_ < 0.001; genotype: F2,65 = 12.8, _P_ < 0.001; interaction: F2,65 = 3.4, _P_ = 0.04) that were blocked in Val/Met and Met/Met mice. No significant effects were observed in (**H**) LMA (effect of NV-5138: F1,64 = 3.39, _P_ > 0.05; genotype: F2,64 = 0.59, P = 0.56; interaction: F2,64 = 0.06, P = 0.94) or (J) HCF (effect of NV-5138: F1,65 = 0.065, P = 0.80; genotype: F2,65 = 0.52, P = 0.60; interaction: F2,65 = 0.58, P = 0.56). Results are shown as mean ± SEM. n = 7–9/group (A–E**); n = 5–16/group (F–J). *P < 0.05; **P < 0.01; ***P < 0.001 (A–J), 2-way ANOVA and Tukey’s post hoc test.
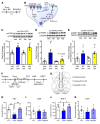
Figure 6. Influence of NV-5138 on spine number and function and synaptic proteins in the PFC.
(A) Representative traces showing postsynaptic currents recorded from layer V pyramidal neurons in mPFC brain slices from vehicle- or NV5138-treated rats (24 hours after drug treatment). (B) Summary of data showing that frequencies of 5-HT– (20 μM) and hypocretin-induced (200 nM) EPSCs are increased by NV-5138; n = 35 cells/8 rats for control; n = 30 cells/8 rats for NV-5138. *P < 0.05; ** P < 0.01, t test. Absolute values for baseline EPSC frequency is 3.58 ± 0.4 (Hz); n = 35 cells/8 rats for control and 2.63 ± 0.48 (Hz); n = 30 cells/8 rats for NV-1538 (C). Representative images of high-magnification Z-stack projections of apical dendritic segments from slices collected 24 hours following vehicle or NV-5138 administration. Scale bar: 5 μm. (D) NV-5138 increased overall spine density (t = 2.72, P = 0.015) due to increases in densities of thin (t = 2.20, P = 0.04) and mushroom spines (t = 2.01, P = 0.05). n = 9 cells/8 rats for control and 9 cells/5 rats for NV-5138. (E–G) Rats were administered vehicle, ketamine (10 mg/kg), or NV-5138 (160 mg/kg), and PFC dissections were collected 24 hours later. Levels of the postsynaptic proteins including (E) GluR1, (F) PSD95, (G) synapsin1, and (H) SV2A were determined by Western blot analysis. GAPDH levels were also determined to control for loading differences. n = 6/group. *P ≤ 0.05; **P < 0.01 Student’s t test.
Figure 7. AMPA receptor antagonist NBQX does not block the antidepressant actions of NV-5138.
(A) Rats were administered either vehicle (0.5% DMSO, 1 ml/kg, i.p.) or NBQX (10 mg/kg, i.p.) 30 minutes prior to administration of vehicle or NV-5138 (160 mg/kg, p.o.). Twenty-four hours after NV-5138 administration, behavioral studies were initiated and performed over the next 3 days. (B and D) NV-5138 treatment significantly decreased immobility time (B) and latency to feed (D), but these effects were not blocked by pretreatment with NBQX in the FST (effect of treatment: F2,21 = 18.5, P < 0.001) and NSFT (effect of treatment: F2,19 = 56.5, _P_ < 0.001), respectively. (**C** and **E**) No significant effects were observed in LMA (effect of treatment: F2,21 = 0.304, _P_ > 0.05) or HCF (effect of treatment: F2,21 = 0.625, P > 0.05). Results are shown as mean ± SEM. n = 7–8/group. ***P < 0.001 compared with control group, 1-way ANOVA followed by post hoc Tukey’s multiple comparison test.
Comment in
- NV-5138 as a fast-acting antidepressant via direct activation of mTORC1 signaling.
Hasegawa Y, Zhu X, Kamiya A. Hasegawa Y, et al. J Clin Invest. 2019 May 20;129(6):2207-2209. doi: 10.1172/JCI129702. eCollection 2019 May 20. J Clin Invest. 2019. PMID: 31107245 Free PMC article.
Similar articles
- Intranasal Administration of Resolvin E1 Produces Antidepressant-Like Effects via BDNF/VEGF-mTORC1 Signaling in the Medial Prefrontal Cortex.
Deyama S, Aoki S, Sugie R, Fukuda H, Shuto S, Minami M, Kaneda K. Deyama S, et al. Neurotherapeutics. 2023 Mar;20(2):484-501. doi: 10.1007/s13311-022-01337-1. Epub 2023 Jan 9. Neurotherapeutics. 2023. PMID: 36622634 Free PMC article. - Activity-dependent brain-derived neurotrophic factor signaling is required for the antidepressant actions of (2_R_,6_R_)-hydroxynorketamine.
Fukumoto K, Fogaça MV, Liu RJ, Duman C, Kato T, Li XY, Duman RS. Fukumoto K, et al. Proc Natl Acad Sci U S A. 2019 Jan 2;116(1):297-302. doi: 10.1073/pnas.1814709116. Epub 2018 Dec 17. Proc Natl Acad Sci U S A. 2019. PMID: 30559184 Free PMC article. - Activity-Dependent Brain-Derived Neurotrophic Factor Release Is Required for the Rapid Antidepressant Actions of Scopolamine.
Ghosal S, Bang E, Yue W, Hare BD, Lepack AE, Girgenti MJ, Duman RS. Ghosal S, et al. Biol Psychiatry. 2018 Jan 1;83(1):29-37. doi: 10.1016/j.biopsych.2017.06.017. Epub 2017 Jun 23. Biol Psychiatry. 2018. PMID: 28751069 Free PMC article. - BDNF - a key transducer of antidepressant effects.
Björkholm C, Monteggia LM. Björkholm C, et al. Neuropharmacology. 2016 Mar;102:72-9. doi: 10.1016/j.neuropharm.2015.10.034. Epub 2015 Nov 11. Neuropharmacology. 2016. PMID: 26519901 Free PMC article. Review. - Pathophysiology of depression and innovative treatments: remodeling glutamatergic synaptic connections.
Duman RS. Duman RS. Dialogues Clin Neurosci. 2014 Mar;16(1):11-27. doi: 10.31887/DCNS.2014.16.1/rduman. Dialogues Clin Neurosci. 2014. PMID: 24733968 Free PMC article. Review.
Cited by
- Imaging the effect of ketamine on synaptic density (SV2A) in the living brain.
Holmes SE, Finnema SJ, Naganawa M, DellaGioia N, Holden D, Fowles K, Davis M, Ropchan J, Emory P, Ye Y, Nabulsi N, Matuskey D, Angarita GA, Pietrzak RH, Duman RS, Sanacora G, Krystal JH, Carson RE, Esterlis I. Holmes SE, et al. Mol Psychiatry. 2022 Apr;27(4):2273-2281. doi: 10.1038/s41380-022-01465-2. Epub 2022 Feb 15. Mol Psychiatry. 2022. PMID: 35165397 Free PMC article. - Intranasal Administration of Resolvin E1 Produces Antidepressant-Like Effects via BDNF/VEGF-mTORC1 Signaling in the Medial Prefrontal Cortex.
Deyama S, Aoki S, Sugie R, Fukuda H, Shuto S, Minami M, Kaneda K. Deyama S, et al. Neurotherapeutics. 2023 Mar;20(2):484-501. doi: 10.1007/s13311-022-01337-1. Epub 2023 Jan 9. Neurotherapeutics. 2023. PMID: 36622634 Free PMC article. - Human retinal ganglion cell axon regeneration by recapitulating developmental mechanisms: effects of recruitment of the mTOR pathway.
Teotia P, Van Hook MJ, Fischer D, Ahmad I. Teotia P, et al. Development. 2019 Jul 4;146(13):dev178012. doi: 10.1242/dev.178012. Development. 2019. PMID: 31273087 Free PMC article. - Excitatory neuron-specific suppression of the integrated stress response contributes to autism-related phenotypes in fragile X syndrome.
Hooshmandi M, Sharma V, Thörn Perez C, Sood R, Krimbacher K, Wong C, Lister KC, Ureña Guzmán A, Bartley TD, Rocha C, Maussion G, Nadler E, Roque PM, Gantois I, Popic J, Lévesque M, Kaufman RJ, Avoli M, Sanz E, Nader K, Hagerman RJ, Durcan TM, Costa-Mattioli M, Prager-Khoutorsky M, Lacaille JC, Martinez-Cerdeno V, Gibson JR, Huber KM, Sonenberg N, Gkogkas CG, Khoutorsky A. Hooshmandi M, et al. Neuron. 2023 Oct 4;111(19):3028-3040.e6. doi: 10.1016/j.neuron.2023.06.017. Epub 2023 Jul 19. Neuron. 2023. PMID: 37473758 Free PMC article. - mTOR at the nexus of nutrition, growth, ageing and disease.
Liu GY, Sabatini DM. Liu GY, et al. Nat Rev Mol Cell Biol. 2020 Apr;21(4):183-203. doi: 10.1038/s41580-019-0199-y. Epub 2020 Jan 14. Nat Rev Mol Cell Biol. 2020. PMID: 31937935 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical