Liver Microphysiological Systems for Predicting and Evaluating Drug Effects - PubMed (original) (raw)
Review
. 2019 Jul;106(1):139-147.
doi: 10.1002/cpt.1458. Epub 2019 Jun 4.
Affiliations
- PMID: 30993668
- PMCID: PMC6771674
- DOI: 10.1002/cpt.1458
Review
Liver Microphysiological Systems for Predicting and Evaluating Drug Effects
Alexandre J S Ribeiro et al. Clin Pharmacol Ther. 2019 Jul.
Abstract
Liver plays a major role in drug metabolism and is one of the main sites of drug adverse effects. Microphysiological systems (MPS), also known as organs-on-a-chip, are a class of microfluidic platforms that recreate properties of tissue microenvironments. Among different properties, the liver microenvironment is three-dimensional, fluid flows around its cells, and different cell types regulate its function. Liver MPS aim to recreate these properties and enable drug testing and measurement of functional endpoints. Tests with these systems have demonstrated their potential for predicting clinical drug effects. Properties of liver MPS that improve the physiology of cell culture are reviewed, specifically focusing on the importance of recreating a physiological microenvironment to evaluate and model drug effects. Advances in modeling hepatic function by leveraging MPS are addressed, noting the need for standardization in the use, quality control, and interpretation of data from these systems.
Published 2019. This article is a U.S. Government work and is in the public domain in the USA. Clinical Pharmacology & Therapeutics published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures
Figure 1
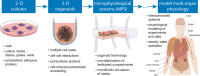
Microphysiological systems (MPS) as advanced platforms to model cellular properties in vitro. Petri dishes and similar two‐dimensional (2‐D) cell culture platforms have been used for almost a century as test beds for maintaining cell cultures and modeling the translation of their biological properties to clinical observations. More recently, spheroid technology has enabled the possibility of simultaneously coculturing different cell types that represent tissue‐specific cellular varieties in three‐dimensions (3‐D) configuration to recreate more physiologically relevant settings in vitro. For developing MPS, organoid‐like coculture approaches were integrated in microfluidic devices with the intent of further improving the physiological relevance of 3‐D cocultures, with the potential to also address several limitations related to lack of human‐specific properties of preclinical tests used in drug development. The field is fast evolving to the deployment of interconnected systems representing interorgan communications and the modeling of more physiologically relevant in vitro assays (63). Illustrations are inspired by different sources.89, 90 [Colour figure can be viewed at
wileyonlinelibrary.com
]
Figure 2
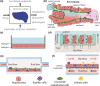
Drug development with liver MPS. Liver microphysiological systems (MPS) result from coculturing different cell types in microfluidic settings that are designed to set the physiology of cellular function. (a) MPS can model drug effects via analysis of metabolism, drug–drug interactions, biomarkers, and phenotypes, such as transport, structure, metabolites, and toxicity. (b) The microenvironment of the liver lobule is multicellular, three‐dimensional, under flow, and defines the sinusoid. (c) two‐dimensional sandwich culture of hepatocytes between a collagen‐coated surface and a layer of Matrigel preserves hepatic function. (d–f) Different designs of liver MPS. Microtissues attach to scaffolds and are exposed to media flow and oxygen gradients in d. Microfluidic chambers (e and f) can also maintain tissue function, under oxygen gradients.91 A porous membrane can separate endothelial cells from hepatocytes to mimic a barrier (f). Illustrations are inspired by different sources.42, 43 [Colour figure can be viewed at
wileyonlinelibrary.com
]
Figure 3
Physiological zonation and cellular activity in the sinusoid unit of the liver. In a healthy and mature liver, oxygen (O2) tension decreases as blood flows along the sinusoid unit (as detailed in Figure 2), from the portal triad (zone 1) to the central vein (zone 3). Different types of cell‐based molecular activities relate to this oxygen tension gradient. Glucose concentration, glycolytic activity, albumin and urea production, Wnt signaling and phosphorylation (phos.) of β‐catenin vary as noted in relation to oxygen concentration. The illustration is inspired by different sources.43, 50 Other cellular functions vary along the hepatic sinusoid, a topic reviewed elsewhere.49, 50, 51 [Colour figure can be viewed at
wileyonlinelibrary.com
]
Figure 4
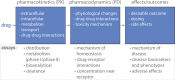
Applications of liver microphysiological systems (MPS) in the field of clinical pharmacology. To understand clinical drug action, studies in clinical pharmacology aim to characterize drug pharmacokinetics, pharmacodynamics, and their relationship to drug effects. For this purpose, different assays can be developed or adapted to liver MPS, and these systems can be used simultaneously to measure, for example, therapeutic and toxic concentrations, characterize metabolism and transporter effects, and evaluate drug–drug interactions. The potential to multiplex different types of measurements in MPS can provide data to support pharmacology models to inform the clinical study design. Pharmacokinetic studies with MPS are also useful for understanding mechanisms that affect drug (and metabolite) concentrations in plasma and liver. [Colour figure can be viewed at
wileyonlinelibrary.com
]
Figure 5
Different possible modes of operation of liver microphysiological systems (MPS) based on circulation of cell culture medium. Developed systems can differ from each other on operational levels that relate to the way cell culture medium is supplied to the cell culture unit. These operational levels have a strong impact in the kinetics of cellular metabolism and energetics due to differences in cellular exposure to nutrients, metabolites, and drugs, which eventually affect system response to drugs.63 (a) Medium can be recirculated and completely changed every 2–3 days throughout the operation time.53 (b) Soluble medium components or compounds can be supplied between media changes.39 (c) Fresh medium can be continuously perfused into the cell culture unit.61 S represents drug substrates, M represents drug metabolites, and X represents carbon sources or other soluble components that set cellular function or physiology. 3‐D, three‐dimensional. [Colour figure can be viewed at
wileyonlinelibrary.com
]
Similar articles
- Characterizing the reproducibility in using a liver microphysiological system for assaying drug toxicity, metabolism, and accumulation.
Rubiano A, Indapurkar A, Yokosawa R, Miedzik A, Rosenzweig B, Arefin A, Moulin CM, Dame K, Hartman N, Volpe DA, Matta MK, Hughes DJ, Strauss DG, Kostrzewski T, Ribeiro AJS. Rubiano A, et al. Clin Transl Sci. 2021 May;14(3):1049-1061. doi: 10.1111/cts.12969. Epub 2021 Apr 3. Clin Transl Sci. 2021. PMID: 33382907 Free PMC article. - A thermoplastic microfluidic microphysiological system to recapitulate hepatic function and multicellular interactions.
Bale SS, Manoppo A, Thompson R, Markoski A, Coppeta J, Cain B, Haroutunian N, Newlin V, Spencer A, Azizgolshani H, Lu M, Gosset J, Keegan P, Charest JL. Bale SS, et al. Biotechnol Bioeng. 2019 Dec;116(12):3409-3420. doi: 10.1002/bit.26986. Epub 2019 Oct 15. Biotechnol Bioeng. 2019. PMID: 30963546 - Fitting tissue chips and microphysiological systems into the grand scheme of medicine, biology, pharmacology, and toxicology.
Watson DE, Hunziker R, Wikswo JP. Watson DE, et al. Exp Biol Med (Maywood). 2017 Oct;242(16):1559-1572. doi: 10.1177/1535370217732765. Exp Biol Med (Maywood). 2017. PMID: 29065799 Free PMC article. - Opportunities and challenges in the wider adoption of liver and interconnected microphysiological systems.
Hughes DJ, Kostrzewski T, Sceats EL. Hughes DJ, et al. Exp Biol Med (Maywood). 2017 Oct;242(16):1593-1604. doi: 10.1177/1535370217708976. Epub 2017 May 15. Exp Biol Med (Maywood). 2017. PMID: 28504617 Free PMC article. Review. - Microphysiological modeling of the reproductive tract: a fertile endeavor.
Eddie SL, Kim JJ, Woodruff TK, Burdette JE. Eddie SL, et al. Exp Biol Med (Maywood). 2014 Sep;239(9):1192-202. doi: 10.1177/1535370214529387. Epub 2014 Apr 15. Exp Biol Med (Maywood). 2014. PMID: 24737736 Free PMC article. Review.
Cited by
- Lipid Fraction from Agaricus brasiliensis as a Potential Therapeutic Agent for Lethal Sepsis in Mice.
Navegantes Lima KC, Gaspar SLF, Oliveira ALB, Santos SMD, Quadros LBG, Oliveira JP, Pereira RCDS, Dias AGDS, Gato LDS, Alencar LYN, Dos Santos ALP, Dorneles GP, Romão PRT, Stutz H, Sovrani V, Monteiro MC. Navegantes Lima KC, et al. Antioxidants (Basel). 2024 Jul 30;13(8):927. doi: 10.3390/antiox13080927. Antioxidants (Basel). 2024. PMID: 39199173 Free PMC article. - Bio inspired heuristic computing scheme for the human liver nonlinear model.
Sabir Z, Ben Said S, Al-Mdallal Q. Sabir Z, et al. Heliyon. 2024 Apr 4;10(7):e28912. doi: 10.1016/j.heliyon.2024.e28912. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 38617930 Free PMC article. - Utility of 1.5 Tesla MRI Scanner in the Management of Small Sample Sizes Driven from 3D Breast Cell Culture.
Guz W, Podgórski R, Aebisher D, Truszkiewicz A, Machorowska-Pieniążek A, Cieślar G, Kawczyk-Krupka A, Bartusik-Aebisher D. Guz W, et al. Int J Mol Sci. 2024 Mar 5;25(5):3009. doi: 10.3390/ijms25053009. Int J Mol Sci. 2024. PMID: 38474256 Free PMC article. - Microphysiological systems as reliable drug discovery and evaluation tools: Evolution from innovation to maturity.
Moon HR, Surianarayanan N, Singh T, Han B. Moon HR, et al. Biomicrofluidics. 2023 Dec 28;17(6):061504. doi: 10.1063/5.0179444. eCollection 2023 Dec. Biomicrofluidics. 2023. PMID: 38162229 Review. - 3D human tissue models and microphysiological systems for HIV and related comorbidities.
3D Human Tissue Models for HIV Working Group. Electronic address: dwight.yin@nih.gov; 3D Human Tissue Models for HIV Working Group. 3D Human Tissue Models for HIV Working Group. Electronic address: dwight.yin@nih.gov, et al. Trends Biotechnol. 2024 May;42(5):526-543. doi: 10.1016/j.tibtech.2023.10.008. Epub 2023 Dec 8. Trends Biotechnol. 2024. PMID: 38071144 Review.
References
- Low, L.A. & Tagle, D.A. Microphysiological systems (tissue chips) and their utility for rare disease research. Adv. Exp. Med. Biol. 1031, 405–415 (2017). - PubMed
- Raasch, M. , Fritsche, E. , Kurtz, A. , Bauer, M. & Mosig, A.S. Microphysiological systems meet hiPSC technology ‐ New tools for disease modeling of liver infections in basic research and drug development. Adv. Drug Deliv. Rev. (2018) 10.1016/j.addr.2018.06.008. [e‐pub ahead of print]. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources