Biological Benefits of Ultra-high Dose Rate FLASH Radiotherapy: Sleeping Beauty Awoken - PubMed (original) (raw)
Review
Biological Benefits of Ultra-high Dose Rate FLASH Radiotherapy: Sleeping Beauty Awoken
M-C Vozenin et al. Clin Oncol (R Coll Radiol). 2019 Jul.
Abstract
FLASH radiotherapy (FLASH-RT) is a technology that could modify the way radiotherapy is delivered in the future. This technique involves the ultra-fast delivery of radiotherapy at dose rates several orders of magnitude higher than those currently used in routine clinical practice. This very short time of exposure leads to the striking observation of relative protection of normal tissues that are exposed to FLASH-RT as compared with conventional dose rate radiotherapy. Here we summarise the current knowledge about the FLASH effect and provide a synthesis of the observations that have been reported on various experimental animal models (mice, zebrafish, pig, cats), various organs (lung, gut, brain, skin) and by various groups across 40 years of research. We also propose possible mechanisms for the FLASH effect, as well as possible paths for clinical application.
Keywords: Differential effect; FLASH radiotherapy; normal tissue protection; oxygen.
Copyright © 2019 The Royal College of Radiologists. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest
The authors declare no conflict of interest.
Figures
Fig 1.
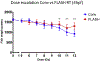
Dose-escalation study. Irradiation was 4 hours post-fertilization (4hpf). Eggs were given 5–12 Gy delivered with FLASH or conventional dose rate irradiation. Radiation-induced alteration of zebrafish morphology was assessed 5 days post-fertilization (5dpf) by body length measurement. FLASH radiotherapy induced lower morphological alterations than conventional radiotherapy at doses above 10 Gy. Mean ± standard deviation and Mann–Whitney’s-test: *P < 0.05; **P < 0.01; ***P < 0.001 (n = 9–19 embryos/group).
Fig 2.
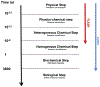
Chronology of physical, physicochemical, chemical, biochemical and biological events occuring after irradiation in the biological tissue. The difference between FLASH radiotherapy and radiotherapy delivered at the conventional dose rate is the duration of the exposure to the ionising radiation during the chemical step of the cascade. The chemical steps are highly dependent on dioxygen concentration within tissues.
Fig 3.
(a) Effect of FLASH in vitro. Representation of isoeffective cell survival to FLASH irradiation in air versus in 0.35% oxygen (~2 mm Hg). The slope in the latter condition becomes parallel to that in nitrogen, i.e. the cells in 0.35% oxygen are depleted to zero oxygen by FLASH irradiation to result in a radioresistant anoxic response. The ratio of the slopes (0.35% oxygen versus air) in this example is ~2.6 and the ratio of isoeffective doses is ~ 30/15 = 2. The difference in these ratios is because of the presence of the breakpoint dose of ~7 Gy needed to deplete the oxygen from 0.35% to zero. The breakpoint dose is higher for higher initial levels of oxygen. Figure based on the results of Nias et al. [22] using Hela cells and 10 MeV linac electrons delivered in a single 1 μs pulse. Very similar results were reported by Berry and Stedeford [6], using P-388 murine leukaemia cell survival assayed in vivo after a single 3 ns pulse from a Febetron-706 400 keV electron generator (see inset graph). 3000 rads = 30 Gy. (b) Effect of FLASH delivery times at 50 (1 μs) pulses per second in vivo. Data taken from [5]. ND50 = dose to produce necrosis in 50% of murine tails by 6 weeks after irradiation. Each data point was derived from two to four experiments; standard errors are ~ ±3% of the mean values plotted. Numbers in circles: tail temperature at the time of irradiation, showing loss of the FLASH effect, presumably due to improved blood flow and higher oxygen tension in the tissue. Numbers in squares: number of (1 μs) pulses per second using the same intrapulse dose rate of 0.4 × 106 Gy/s, showing loss of the FLASH effect with protraction of overall dose delivery.
Fig 4.
Average lung reaction scores per dose group = sum of percentage of animals × 1, 2, 3, 4 unitised scores of ±, +, ++, +++, read from [9, figure 1]. C,D, conventional dose rate, assay at 24 and 36 weeks; F,G, FLASH dose rate, assay at 24 and 36 weeks. Dashed lines are drawn for visual trend, no strict linear model is implied.
Comment in
- FLASH Radiotherapy: The Next Technological Advance in Radiation Therapy?
Symonds P, Jones GDD. Symonds P, et al. Clin Oncol (R Coll Radiol). 2019 Jul;31(7):405-406. doi: 10.1016/j.clon.2019.05.011. Clin Oncol (R Coll Radiol). 2019. PMID: 31178010 No abstract available.
Similar articles
- FLASH Radiotherapy: Benefits, Mechanisms, and Obstacles to Its Clinical Application.
Alhaddad L, Osipov AN, Leonov S. Alhaddad L, et al. Int J Mol Sci. 2024 Nov 21;25(23):12506. doi: 10.3390/ijms252312506. Int J Mol Sci. 2024. PMID: 39684218 Free PMC article. Review. - The Advantage of FLASH Radiotherapy Confirmed in Mini-pig and Cat-cancer Patients.
Vozenin MC, De Fornel P, Petersson K, Favaudon V, Jaccard M, Germond JF, Petit B, Burki M, Ferrand G, Patin D, Bouchaab H, Ozsahin M, Bochud F, Bailat C, Devauchelle P, Bourhis J. Vozenin MC, et al. Clin Cancer Res. 2019 Jan 1;25(1):35-42. doi: 10.1158/1078-0432.CCR-17-3375. Epub 2018 Jun 6. Clin Cancer Res. 2019. PMID: 29875213 - [A New Generation of Radiotherapy Technology-Flash Radiotherapy].
Wu C, Song J, Yin B, Zhang G, Lin H, Fang C, Yang T, Qu B, Xu S. Wu C, et al. Zhongguo Yi Liao Qi Xie Za Zhi. 2020 Dec 8;44(6):508-512. doi: 10.3969/j.issn.1671-7104.2020.06.009. Zhongguo Yi Liao Qi Xie Za Zhi. 2020. PMID: 33314859 Chinese. - FLASH Radiotherapy: Current Knowledge and Future Insights Using Proton-Beam Therapy.
Hughes JR, Parsons JL. Hughes JR, et al. Int J Mol Sci. 2020 Sep 5;21(18):6492. doi: 10.3390/ijms21186492. Int J Mol Sci. 2020. PMID: 32899466 Free PMC article. Review. - Can Rational Combination of Ultra-high Dose Rate FLASH Radiotherapy with Immunotherapy Provide a Novel Approach to Cancer Treatment?
Zhang Y, Ding Z, Perentesis JP, Khuntia D, Pfister SX, Sharma RA. Zhang Y, et al. Clin Oncol (R Coll Radiol). 2021 Nov;33(11):713-722. doi: 10.1016/j.clon.2021.09.003. Epub 2021 Sep 20. Clin Oncol (R Coll Radiol). 2021. PMID: 34551871 Review.
Cited by
- Oxygen supplementation in anesthesia can block FLASH effect and anti-tumor immunity in conventional proton therapy.
Iturri L, Bertho A, Lamirault C, Brisebard E, Juchaux M, Gilbert C, Espenon J, Sébrié C, Jourdain L, de Marzi L, Pouzoulet F, Muret J, Verrelle P, Prezado Y. Iturri L, et al. Commun Med (Lond). 2023 Dec 15;3(1):183. doi: 10.1038/s43856-023-00411-9. Commun Med (Lond). 2023. PMID: 38102219 Free PMC article. - An efficient rectangular optimization method for sparse orthogonal collimator based small animal irradiation.
Jiang L, Lyu Q, Abdelhamid AMH, Hui S, Sheng K. Jiang L, et al. Phys Med Biol. 2022 Sep 28;67(19):10.1088/1361-6560/ac910b. doi: 10.1088/1361-6560/ac910b. Phys Med Biol. 2022. PMID: 36084625 Free PMC article. - A human lung alveolus-on-a-chip model of acute radiation-induced lung injury.
Dasgupta Q, Jiang A, Wen AM, Mannix RJ, Man Y, Hall S, Javorsky E, Ingber DE. Dasgupta Q, et al. Nat Commun. 2023 Oct 16;14(1):6506. doi: 10.1038/s41467-023-42171-z. Nat Commun. 2023. PMID: 37845224 Free PMC article. - Non-Surgical Definitive Treatment for Operable Breast Cancer: Current Status and Future Prospects.
Shibamoto Y, Takano S. Shibamoto Y, et al. Cancers (Basel). 2023 Mar 20;15(6):1864. doi: 10.3390/cancers15061864. Cancers (Basel). 2023. PMID: 36980750 Free PMC article. Review. - Implementation and validation of a beam-current transformer on a medical pulsed electron beam LINAC for FLASH-RT beam monitoring.
Oesterle R, Gonçalves Jorge P, Grilj V, Bourhis J, Vozenin MC, Germond JF, Bochud F, Bailat C, Moeckli R. Oesterle R, et al. J Appl Clin Med Phys. 2021 Nov;22(11):165-171. doi: 10.1002/acm2.13433. Epub 2021 Oct 5. J Appl Clin Med Phys. 2021. PMID: 34609051 Free PMC article.
References
- Bristow RG, Alexander B, Baumann M, Bratman SV, Brown JM, Camphausen K, et al. Combining precision radiotherapy with molecular targeting and immunomodulatory agents: a guideline by the American Society for Radiation Oncology. Lancet Oncol 2018;19(5):e240–e251. 10.1016/S1470-2045(18)30096-2. - DOI - PubMed
- Hornsey S, Bewley DK. Hypoxia in mouse intestine induced by electron irradiation at high dose-rates. Int J Radiat Biol Relat Stud Phys Chem Med 1971;19(5):479–483. - PubMed
- Field SB, Bewley DK. Effects of dose-rate on the radiation response of rat skin. Int J Radiat Biol Relat Stud Phys Chem Med 1974;26(3):259–267. - PubMed
- Hendry JH, Moore JV, Hodgson BW, Keene JP. The constant low oxygen concentration in all the target cells for mouse tail radionecrosis. Radiat Res 1982;92(1):172–181. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous