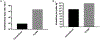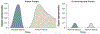An integrated physico-chemical approach for explaining the differential impact of FLASH versus conventional dose rate irradiation on cancer and normal tissue responses - PubMed (original) (raw)
An integrated physico-chemical approach for explaining the differential impact of FLASH versus conventional dose rate irradiation on cancer and normal tissue responses
Douglas R Spitz et al. Radiother Oncol. 2019 Oct.
Abstract
For decades the field of radiation oncology has sought to improve the therapeutic ratio through innovations in physics, chemistry, and biology. To date, technological advancements in image guided beam delivery techniques have provided clinicians with their best options for improving this critical tool in cancer care. Medical physics has focused on the preferential targeting of tumors while minimizing the collateral dose to the surrounding normal tissues, yielding only incremental progress. However, recent developments involving ultra-high dose rate irradiation termed FLASH radiotherapy (FLASH-RT), that were initiated nearly 50 years ago, have stimulated a renaissance in the field of radiotherapy, long awaiting a breakthrough modality able to enhance therapeutic responses and limit normal tissue injury. Compared to conventional dose rates used clinically (0.1-0.2 Gy/s), FLASH can implement dose rates of electrons or X-rays in excess of 100 Gy/s. The implications of this ultra-fast delivery of dose are significant and need to be re-evaluated to appreciate the fundamental aspects underlying this seemingly unique radiobiology. The capability of FLASH to significantly spare normal tissue complications in multiple animal models, when compared to conventional rates of dose-delivery, while maintaining persistent growth inhibition of select tumor models has generated considerable excitement, as well as skepticism. Based on fundamental principles of radiation physics, radio-chemistry, and tumor vs. normal cell redox metabolism, this article presents a series of testable, biologically relevant hypotheses, which may help rationalize the differential effects of FLASH irradiation observed between normal tissue and tumors.
Keywords: FLASH radiation; Free radical chemistry; Organic hydroperoxides; Oxygen; Redox active iron; Tumor versus normal tissue responses.
Copyright © 2019 Elsevier B.V. All rights reserved.
Conflict of interest statement
The Authors of this manuscript declare no conflict of interest
Figures
Figure 1.
Graphical representation of theoretical results comparing an instantaneous pulse from a conventional and FLASH electron beam. (A) instantaneous dose rate; and (B) number of ionizations produced following an instantaneous pulse (ionizations kg−1).
Figure 2.
Proposed mechanism for differential dissipation of organic hydroperoxide levels in FLASH vs. conventional radiation damage to normal versus tumor tissue.
Comment in
- Re: Differential impact of FLASH versus conventional dose rate irradiation: Spitz et al.
Koch CJ. Koch CJ. Radiother Oncol. 2019 Oct;139:62-63. doi: 10.1016/j.radonc.2019.07.004. Epub 2019 Aug 17. Radiother Oncol. 2019. PMID: 31431380 No abstract available. - To the Editors.
Ling CC, Josef G. Ling CC, et al. Radiother Oncol. 2020 Jun;147:240. doi: 10.1016/j.radonc.2020.02.027. Epub 2020 Mar 24. Radiother Oncol. 2020. PMID: 32220508 No abstract available. - Response to Ling et al. regarding "An integrated physico-chemical approach for explaining the differential impact of FLASH versus conventional dose rate irradiation on cancer and normal tissue responses".
Buettner GR, Spitz DR, Limoli CL. Buettner GR, et al. Radiother Oncol. 2020 Jun;147:241-242. doi: 10.1016/j.radonc.2020.03.001. Epub 2020 Mar 25. Radiother Oncol. 2020. PMID: 32222332 Free PMC article. No abstract available.
Similar articles
- Understanding the FLASH effect to unravel the potential of ultra-high dose rate irradiation.
Kacem H, Almeida A, Cherbuin N, Vozenin MC. Kacem H, et al. Int J Radiat Biol. 2022;98(3):506-516. doi: 10.1080/09553002.2021.2004328. Epub 2021 Dec 2. Int J Radiat Biol. 2022. PMID: 34788193 Review. - FLASH ultra-high dose rates in radiotherapy: preclinical and radiobiological evidence.
Borghini A, Vecoli C, Labate L, Panetta D, Andreassi MG, Gizzi LA. Borghini A, et al. Int J Radiat Biol. 2022;98(2):127-135. doi: 10.1080/09553002.2022.2009143. Epub 2021 Dec 16. Int J Radiat Biol. 2022. PMID: 34913413 Review. - Potential Molecular Mechanisms behind the Ultra-High Dose Rate "FLASH" Effect.
Bogaerts E, Macaeva E, Isebaert S, Haustermans K. Bogaerts E, et al. Int J Mol Sci. 2022 Oct 11;23(20):12109. doi: 10.3390/ijms232012109. Int J Mol Sci. 2022. PMID: 36292961 Free PMC article. Review. - Radiobiology of the FLASH effect.
Friedl AA, Prise KM, Butterworth KT, Montay-Gruel P, Favaudon V. Friedl AA, et al. Med Phys. 2022 Mar;49(3):1993-2013. doi: 10.1002/mp.15184. Epub 2021 Sep 20. Med Phys. 2022. PMID: 34426981 - The Importance and Clinical Implications of FLASH Ultra-High Dose-Rate Studies for Proton and Heavy Ion Radiotherapy.
Colangelo NW, Azzam EI. Colangelo NW, et al. Radiat Res. 2020 Jan;193(1):1-4. doi: 10.1667/RR15537.1. Epub 2019 Oct 28. Radiat Res. 2020. PMID: 31657670 Free PMC article.
Cited by
- Emergence of FLASH‑radiotherapy across the last 50 years (Review).
Li M, Zhou S, Dong G, Wang C. Li M, et al. Oncol Lett. 2024 Oct 11;28(6):602. doi: 10.3892/ol.2024.14735. eCollection 2024 Dec. Oncol Lett. 2024. PMID: 39493433 Free PMC article. Review. - FLASH Radiotherapy: Mechanisms of Biological Effects and the Therapeutic Potential in Cancer.
Yan O, Wang S, Wang Q, Wang X. Yan O, et al. Biomolecules. 2024 Jun 25;14(7):754. doi: 10.3390/biom14070754. Biomolecules. 2024. PMID: 39062469 Free PMC article. Review. - A potential revolution in cancer treatment: A topical review of FLASH radiotherapy.
Gao Y, Liu R, Chang CW, Charyyev S, Zhou J, Bradley JD, Liu T, Yang X. Gao Y, et al. J Appl Clin Med Phys. 2022 Oct;23(10):e13790. doi: 10.1002/acm2.13790. Epub 2022 Sep 27. J Appl Clin Med Phys. 2022. PMID: 36168677 Free PMC article. Review. - Radical Production with Pulsed Beams: Understanding the Transition to FLASH.
Espinosa-Rodriguez A, Sanchez-Parcerisa D, Ibáñez P, Vera-Sánchez JA, Mazal A, Fraile LM, Manuel Udías J. Espinosa-Rodriguez A, et al. Int J Mol Sci. 2022 Nov 3;23(21):13484. doi: 10.3390/ijms232113484. Int J Mol Sci. 2022. PMID: 36362271 Free PMC article. - Bridging Radiotherapy to Immunotherapy: The IFN-JAK-STAT Axis.
Shi LZ, Bonner JA. Shi LZ, et al. Int J Mol Sci. 2021 Nov 14;22(22):12295. doi: 10.3390/ijms222212295. Int J Mol Sci. 2021. PMID: 34830176 Free PMC article.
References
- Favaudon V, Caplier L, Monceau V, Pouzoulet F, Sayarath M, Fouillade C, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med. 2014;6:245ra93. - PubMed
- Vozenin MC, De Fornel P, Petersson K, Favaudon V, Jaccard M, Germond JF, et al. The Advantage of FLASH Radiotherapy Confirmed in Mini-pig and Cat-cancer Patients. Clin Cancer Res. 2019;25:35–42. - PubMed
- Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: a unifying concept in stress response biology. Cancer Metastasis Rev. 2004;23:311–22. - PubMed
Publication types
MeSH terms
Grants and funding
- P30 CA086862/CA/NCI NIH HHS/United States
- R01 CA169046/CA/NCI NIH HHS/United States
- R01 NS089575/NS/NINDS NIH HHS/United States
- T32 CA078586/CA/NCI NIH HHS/United States
- R01 CA182804/CA/NCI NIH HHS/United States
- P50 CA174521/CA/NCI NIH HHS/United States
- P01 CA217797/CA/NCI NIH HHS/United States
- P30 ES005605/ES/NIEHS NIH HHS/United States