Molecular Mechanisms of Dermal Aging and Antiaging Approaches - PubMed (original) (raw)
Review
Molecular Mechanisms of Dermal Aging and Antiaging Approaches
Jung-Won Shin et al. Int J Mol Sci. 2019.
Abstract
The dermis is primarily composed of the extracellular matrix (ECM) and fibroblasts. During the aging process, the dermis undergoes significant changes. Collagen, which is a major component of ECM, becomes fragmented and coarsely distributed, and its total amount decreases. This is mainly due to increased activity of matrix metalloproteinases, and impaired transforming growth factor-β signaling induced by reactive oxygen species generated during aging. The reduction in the amount of collagen hinders the mechanical interaction between fibroblasts and the ECM, and consequently leads to the deterioration of fibroblast function and further decrease in the amount of dermal collagen. Other ECM components, including elastic fibers, glycosaminglycans (GAGs), and proteoglycans (PGs), also change during aging, ultimately leading to a reduction in the amount of functional components. Elastic fibers decrease in intrinsically aged skin, but accumulate abnormally in photoaged skin. The changes in the levels of GAGs and PGs are highly diverse, and previous studies have reported conflicting results. A reduction in the levels of functional dermal components results in the emergence of clinical aging features, such as wrinkles and reduced elasticity. Various antiaging approaches, including topicals, energy-based procedures, and dermal fillers, can restore the molecular features of dermal aging with clinical efficacy. This review summarizes the current understanding of skin aging at the molecular level, and associated treatments, to put some of the new antiaging technology that has emerged in this rapidly expanding field into molecular context.
Keywords: collagen; dermal aging; elastic fiber; fibroblast; glycosaminglycans; hyaluronic acid; proteoglycans.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
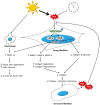
Figure 1
Schematic illustration showing the changes in fibroblasts, collagen, and elastic fibers in the dermal aging process. Reactive oxygen species (ROS) generated in the aging process activate mitogen-activated protein kinases (MAPKs) and induce transcription factors, including activator protein 1 (AP-1) and nuclear factor-κB (NF-κB). This activation increases matrix metalloproteinase (MMP) expression and inhibits transforming growth factor-β (TGF-β) signaling, which leads to collagen fragmentation and decreased collagen biosynthesis. This hinders the mechanical interaction between fibroblasts and the extracellular matrix (ECM), and consequently reduces the size of dermal fibroblasts. Aged fibroblasts produce a greater amount of ROS that further increases the expression of MMPs and inhibits TGF-β signaling, creating a positive feedback loop that accelerates dermal aging. MMP-12 secreted from fibroblasts and macrophages plays a crucial role in the development of solar elastosis and in the reduction of functional elastic fibers.
Similar articles
- Rejuvenation of Aged Human Skin by Injection of Cross-linked Hyaluronic Acid.
Cui Y, Wang F, Voorhees JJ, Fisher GJ. Cui Y, et al. Plast Reconstr Surg. 2021 Jan 1;147(1S-2):43S-49S. doi: 10.1097/PRS.0000000000007620. Plast Reconstr Surg. 2021. PMID: 33347074 - Anti‑apoptotic effects of glycosaminoglycans via inhibition of ERK/AP‑1 signaling in TNF‑α‑stimulated human dermal fibroblasts.
Na J, Bak DH, Im SI, Choi H, Hwang JH, Kong SY, No YA, Lee Y, Kim BJ. Na J, et al. Int J Mol Med. 2018 May;41(5):3090-3098. doi: 10.3892/ijmm.2018.3483. Epub 2018 Feb 12. Int J Mol Med. 2018. PMID: 29436595 - Characterization and mechanisms of photoageing-related changes in skin. Damages of basement membrane and dermal structures.
Amano S. Amano S. Exp Dermatol. 2016 Aug;25 Suppl 3:14-9. doi: 10.1111/exd.13085. Exp Dermatol. 2016. PMID: 27539897 Review. - Age-related reduction of dermal fibroblast size upregulates multiple matrix metalloproteinases as observed in aged human skin in vivo.
Qin Z, Balimunkwe RM, Quan T. Qin Z, et al. Br J Dermatol. 2017 Nov;177(5):1337-1348. doi: 10.1111/bjd.15379. Epub 2017 Nov 1. Br J Dermatol. 2017. PMID: 28196296 Free PMC article. - Glycosaminoglycan and proteoglycan in skin aging.
Lee DH, Oh JH, Chung JH. Lee DH, et al. J Dermatol Sci. 2016 Sep;83(3):174-81. doi: 10.1016/j.jdermsci.2016.05.016. Epub 2016 May 27. J Dermatol Sci. 2016. PMID: 27378089 Review.
Cited by
- iTRAQ-Based Proteomic Profiling of Skin Aging Protective Effects of _Tremella fuciformis_-Derived Polysaccharides on D-Galactose-Induced Aging Mice.
Xu Y, Liu X, Guan J, Chen J, Xu X. Xu Y, et al. Molecules. 2024 Nov 2;29(21):5191. doi: 10.3390/molecules29215191. Molecules. 2024. PMID: 39519833 Free PMC article. - Exploring the Potential of Anthocyanins for Repairing Photoaged Skin: A Comprehensive Review.
Guo X, He L, Sun J, Ye H, Yin C, Zhang W, Han H, Jin W. Guo X, et al. Foods. 2024 Nov 1;13(21):3506. doi: 10.3390/foods13213506. Foods. 2024. PMID: 39517290 Free PMC article. Review. - Facial aging, cognitive impairment, and dementia risk.
Xu X, Jigeer G, Gunn DA, Liu Y, Chen X, Guo Y, Li Y, Gu X, Ma Y, Wang J, Wang S, Sun L, Lin X, Gao X. Xu X, et al. Alzheimers Res Ther. 2024 Nov 6;16(1):245. doi: 10.1186/s13195-024-01611-8. Alzheimers Res Ther. 2024. PMID: 39506848 Free PMC article. - Skin senescence-from basic research to clinical practice.
Dorf N, Maciejczyk M. Dorf N, et al. Front Med (Lausanne). 2024 Oct 18;11:1484345. doi: 10.3389/fmed.2024.1484345. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39493718 Free PMC article. Review. - Isopimpinellin inhibits UVA-induced overproduction of MMPs via suppression of MAPK/AP-1 signaling in human dermal fibroblasts.
Oh JH, Karadeniz F, Seo Y, Kong CS. Oh JH, et al. Food Sci Biotechnol. 2024 Jul 24;33(15):3579-3589. doi: 10.1007/s10068-024-01611-2. eCollection 2024 Dec. Food Sci Biotechnol. 2024. PMID: 39493386
References
- Varani J., Dame M.K., Rittie L., Fligiel S.E., Kang S., Fisher G.J., Voorhees J.J. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006;168:1861–1868. doi: 10.2353/ajpath.2006.051302. - DOI - PMC - PubMed
- Xia W., Quan T., Hammerberg C., Voorhees J.J., Fisher G.J. A mouse model of skin aging: Fragmentation of dermal collagen fibrils and reduced fibroblast spreading due to expression of human matrix metalloproteinase-1. J. Dermatol. Sci. 2015;78:79–82. doi: 10.1016/j.jdermsci.2015.01.009. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical