Anaerobic Glycolysis Maintains the Glomerular Filtration Barrier Independent of Mitochondrial Metabolism and Dynamics - PubMed (original) (raw)
. 2019 Apr 30;27(5):1551-1566.e5.
doi: 10.1016/j.celrep.2019.04.012.
Tillmann Bork 2, Sarah Salou 2, Wei Liang 2, Athanasia Mizi 1, Cem Özel 1, Sybille Koehler 1, H Henning Hagmann 1, Christina Ising 1, Alexander Kuczkowski 1, Svenia Schnyder 3, Ahmed Abed 2, Bernhard Schermer 1, Thomas Benzing 1, Oliver Kretz 4, Victor G Puelles 5, Simon Lagies 6, Manuel Schlimpert 6, Bernd Kammerer 7, Christoph Handschin 3, Christoph Schell 8, Tobias B Huber 9
Affiliations
- PMID: 31042480
- PMCID: PMC6506687
- DOI: 10.1016/j.celrep.2019.04.012
Anaerobic Glycolysis Maintains the Glomerular Filtration Barrier Independent of Mitochondrial Metabolism and Dynamics
Paul T Brinkkoetter et al. Cell Rep. 2019.
Abstract
The cellular responses induced by mitochondrial dysfunction remain elusive. Intrigued by the lack of almost any glomerular phenotype in patients with profound renal ischemia, we comprehensively investigated the primary sources of energy of glomerular podocytes. Combining functional measurements of oxygen consumption rates, glomerular metabolite analysis, and determination of mitochondrial density of podocytes in vivo, we demonstrate that anaerobic glycolysis and fermentation of glucose to lactate represent the key energy source of podocytes. Under physiological conditions, we could detect neither a developmental nor late-onset pathological phenotype in podocytes with impaired mitochondrial biogenesis machinery, defective mitochondrial fusion-fission apparatus, or reduced mtDNA stability and transcription caused by podocyte-specific deletion of Pgc-1α, Drp1, or Tfam, respectively. Anaerobic glycolysis represents the predominant metabolic pathway of podocytes. These findings offer a strategy to therapeutically interfere with the enhanced podocyte metabolism in various progressive kidney diseases, such as diabetic nephropathy or focal segmental glomerulosclerosis (FSGS).
Keywords: anaerobic glycolysis; glomerular filtration barrier; metabolomics; podocytes.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Graphical abstract
Figure 1
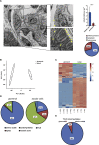
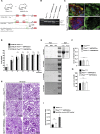
Podocytes Display Low Mitochondrial Density (A) Image obtained by transmission electron microscopy (TEM) displaying a glomerulus with neighboring tubular cells. Boxed areas show details from a podocyte and a proximal tubular cell, respectively. Mitochondria are marked by arrowheads. The depicted image is stitched from a 3×3 array of single images. Quantification of mitochondrial density and distribution of mitochondria in podocytes (based on the analysis of 70 pictures obtained from 8 mice; ∗∗∗p ≤ 0.001; presented as mean ± SD). (B) Principal-component analysis (PCA) of metabolite content of glomeruli and the tubular compartment (obtained from 4 mice each). (C) Heatmap of metabolites detected in glomeruli and in the tubular compartment (obtained from 4 mice each). (D) Metabolite composition of glomerular and tubular samples based on superclasses of metabolites. (E) Abundance of intermediates of tricarboxylic acid (TCA) cycle in glomeruli and the tubular compartment.
Figure 2
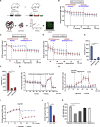
Primary Podocytes Rely on Glycolysis (A) Primary podocyte culture was obtained by crossing hNPHS2Cre mice with a Tomato/EGFP reporter strain. Schematic of glomerular culture followed by fluorescence-activated cell sorting (FACS) is shown. (B) Dependency and capacity of fatty acid oxidation obtained after etomoxir and BPTESm UK5099 injection or BPTES, UK5099 followed by etomoxir, respectively (mean ± SD). Final concentrations: etomoxir: 100 μM; BPTES: 30 μM; UK5099: 30 μM. (C) Dependency and capacity of glutamine oxidation obtained after BPTES and etomoxir, UK5099 or etomoxir, UK5099 followed by BPTES, respectively (mean ± SD). Final concentrations: etomoxir: 100 μM; BPTES: 30 μM; UK5099: 30 μM. (D) Dependency and capacity of pyruvate oxidation obtained after UK5099 and etomoxir, BPTES and etomoxir, BPTES followed by UK5099, respectively (mean ± SD). Final concentrations: etomoxir: 100 μM; BPTES: 30 μM; UK5099: 30 μM. (E) Statistics displaying substrate dependency of primary podocytes (data out of 3 experiments; ∗p ≤ 0.05; ∗∗p ≤ 0.01; mean ± SD). (F) Statistics displaying substrate capacity of primary podocytes (data out of 3 experiments; ∗p ≤ 0.05; ∗∗p ≤ 0.01; mean ± SD). (G) Oxygen consumption rate (OCR) of primary podocytes after injection of 2-deoxyglucose or vehicle followed by the inhibitors of the respiratory chain as indicated (mean ± SD). Final concentration: 2-DG: 50 mM; oligomycin: 1 μM; FCCP: 0.5 μM; rotenone: 0.5 μM; antimycin: 0.5 μM. (H) Extracellular acidification rate (ECAR) of primary podocytes obtained after injection of glucose or vehicle followed by oligomycin and 2-DG as indicated (mean ± SD). Final concentration: 2-DG: 50 mM; oligomycin: 1 μM; rotenone: 0.5 μM; antimycin: 0.5 μM. (I) ECAR of primary podocytes after oxamate or vehicle injection (mean ± SD). Final concentration: oxamate: 45 mM. (J) Statistics displaying ECAR after vehicle or oxamate injection (∗∗∗p ≤ 0.001; mean ± SD). (K) Statistics showing intracellular ATP content after 24-h treatment with 2-DG, oxamate, etomoxir, or oligomycin (∗p ≤ 0.05; ∗∗ p ≤ 0.01; mean ± SD). Final concentration: 2-DG: 50 mM; oxamate: 45 mM; etomoxir: 100 μM; oligomycin: 1 μM.
Figure 3
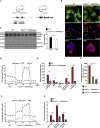
Loss of DRP1 Does Not Affect Glomerular Function and Morphology (A) Schematic illustrating targeted gene deletion approach of Drp1 in podocytes using the Cre-loxP system. (B) Breeding strategy to generate a podocyte-based reporter strain. After isolation of GFP+ podocytes via FACS, DNA was prepared and respective genotypes were verified using the following primers: D1 (5′-CACTGAGAGCTCTATATGTAGGC-3′); D3 (5′-ACCAAAGTAAGGAATAGCTGTTG-3′); and D5 (5′-GAGTACCTAAAGTGGACAAGAGGTCC-3′). PCR products were detected at 315 bp and 539 bp. (C) Western blot analysis confirmed reduced DRP1 levels in glomeruli of conditional Drp1 flox/flox ∗hNPHS2Cre mice. (D) Densitometric quantification of DRP1 protein levels demonstrated a significant reduction in glomeruli derived from KO animals (n = 3; ∗p ≤ 0.05; mean ± SD). (E) Immunofluorescence stainings visualized absence of DRP1 in knockout podocytes. Dotted lines indicate the respective podocyte compartment, as marked with NEPHRIN staining. (F) 12-month follow-up period revealed no differences in body weight between wild-type and Drp1 knockout animals (at least 3 animals per genotype and time point were analyzed; mean ± SD). (G) Measurement of ACR (albumin-to-creatinine ratio) revealed no differences during the 12-month follow-up period (at least 3 animals per genotype and time point were analyzed; mean ± SD). (H) Periodic acid Schiff (PAS)-stained kidney sections obtained from 1-month-old and 12-month-old Drp1 flox/flox ∗hNPHS2Cre mice and littermate controls showed normal glomerular structure.
Figure 4
Mitochondria Are Elongated in _Drp1_-Deficient Podocytes Representative TEM pictures displaying mitochondrial shape and density in podocytes. Sections were obtained from mouse kidneys at the age of 1 month, 5 months, and 7 months, respectively.
Figure 5
Podocyte-Specific _Pgc-1_α Knockout Mice Are Viable and Do Not Display a Glomerular Phenotype (A) Schematic illustrating targeted gene deletion approach of Pgc-1α in podocytes using the Cre-loxP system. (B) After isolation of podocytes via antibody-based labeling followed by FACS, DNA was prepared and respective genotypes were verified using the following primers: Pgc-1α WT: forward 5′-ACCTGTCTTTGCCTATGATTC-3′, reverse CCAGTTTCTTCATTGGTGTG; Pgc-1α KO forward 5′-TCCAGTAGGCAGAGATTTATGAC-3′, reverse 5′-CCAACTGTCTATAATTCCAGTTC-3′. (C) In a 22-month follow-up period, no differences in body weight were detected between wild-type and Pgc-1α knockout animals (at least 3 animals per genotype and time point were analyzed; mean ± SD). (D) Measurement of ACR revealed no differences during the 22-month follow-up period (at least 3 animals per genotype and time point were analyzed; mean ± SD). (E) PAS-stained kidney sections from 3-month-old and 22-month-old Pgc-1α flox/flox_∗_hNPHS2Cre mice and littermate controls showed normal glomerular structure. (F) TEM images obtained from 22-month-old Pgc-1α flox/flox ∗hNPHS2Cre and littermate controls show normal foot process formation and no difference in mitochondrial shape (representative pictures).
Figure 6
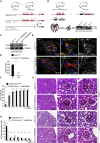
Podocyte-Specific Tfam Knockout Mice Do Not Develop Glomerular Disease (A) Schematic illustrating targeted gene deletion approach of Tfam in podocytes using the Cre-loxP system. (B) After isolation of glomeruli, DNA was prepared and respective genotypes were verified using following primers: Tfam forward 5′-CTGCCTTCCTAGCCCGG-3′; Tfam reverse 1 5′-GTAACAGCAGACAACTTGTG-3′; Tfam reverse 2 5′-CTCTGAAGCACATGGTCAAT-3′. (C) Immunofluorescence staining for TFAM in kidney sections obtained from 3-month-old Tfam flox/flox ∗hNPHS2Cre mice and respective controls. (D) 12-month follow-up period revealed no differences in body weight between wild-type, Tfam flox/+ ∗hNPHS2Cre mice and Tfam flox/flox ∗hNPHS2Cre mice (at least 3 animals per genotype and time point were analyzed; mean ± SD). (E) Urinary protein excretion was absent during a 12-month follow-up period in Tfam flox/flox , Tfam flox/+ ∗hNPHS2Cre mice and Tfam flox/flox ∗hNPHS2Cre, respectively, as indicated by representative Coomassie brilliant blue staining. (F) Serum creatinine levels in 6-month-old Tfam flox/flox , Tfam flox/+ ∗hNPHS2Cre mice and Tfam flox/flox ∗hNPHS2Cre mice, respectively (n = 4 each genotype; mean ± SD). (G) Serum urea levels in 6-month-old Tfam flox/flox , Tfam flox/+ ∗hNPHS2Cre mice and Tfam flox/flox ∗hNPHS2Cre mice, respectively (n = 4 each genotype; mean ± SD). (H) PAS-stained kidney sections obtained from 55-week-old Tfam flox/flox , Tfam flox/+ ∗hNPHS2Cre mice and Tfam flox/flox ∗hNPHS2Cre mice, respectively. (I) Glomerulosclerosis assessment in kidney sections obtained from 55-week-old Tfam flox/flox and Tfam flox/flox ∗hNPHS2Cre mice, as proposed by el Nahas et al. (1991). Adriamycin-induced nephropathy in WT mice (ICR-CD1 background sacrificed 50 days after induction) served as positive control (n = 3 each group; 100 glomeruli were assessed per mouse; mean ± SD).
Figure 7
Metabolic Profiling of _Tfam_-Deficient Podocytes Revealed Decreased Respiratory Activity (A) Schematic illustrating breeding strategy to generate primary podocytes lacking Tfam. (B) Immunofluorescence image obtained from primary podocytes deficient for Tfam and controls shows a comparable distribution and intensity for the mitochondrial marker TOMM20 (red). (C) Western blot analysis for key components of the respiratory chain revealed lower levels of MTCO1 in _Tfam_-deficient podocytes (n = 4; ∗p ≤ 0.05; mean ± SD). (D) OCR from _Tfam_-deficient podocytes and controls at baseline and after subsequent injection of oligomycin, FCCP, and rotenone, antimycin A (mean ± SD). Final concentration: oligomycin: 1 μM; FCCP: 0.5 μM; rotenone: 0.5 μM; antimycin: 0.5 μM. (E) Statistics of key parameters of respiratory function in _Tfam_-deficient podocytes and controls (∗p ≤ 0.05; ∗∗p ≤ 0.01; mean ± SD). (F) Quantitative CM-H2DCFDA fluorescence obtained from primary podocytes with complete or heterozygous disruption of Tfam and controls, indicating oxidative stress (∗∗p ≤ 0.01; ∗∗∗p ≤ 0.001; mean ± SD). (G) Extracellular acidification rate of _Tfam_-deficient podocytes and respective controls followed after glucose administration and subsequent injection of oligomycin and 2-DG (mean ± SD). Final concentration: glucose: 10 mM; oligomycin: 1 μM; 2-DG: 50 mM. (H) Statistics of key parameters of glycolytic activity obtained from _Tfam_-deficient podocytes and respective controls (∗p ≤ 0.05; ∗∗p ≤ 0.01; mean ± SD).
References
- Baris O.R., Klose A., Kloepper J.E., Weiland D., Neuhaus J.F., Schauen M., Wille A., Müller A., Merkwirth C., Langer T. The mitochondrial electron transport chain is dispensable for proliferation and differentiation of epidermal progenitor cells. Stem Cells. 2011;29:1459–1468. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous