Emerging class of omega-3 fatty acid endocannabinoids & their derivatives - PubMed (original) (raw)
Review
Emerging class of omega-3 fatty acid endocannabinoids & their derivatives
Josephine E Watson et al. Prostaglandins Other Lipid Mediat. 2019 Aug.
Abstract
Cannabinoid receptor activation is involved in homeostatic regulation of the body. These receptors are activated by cannabinoids, that include the active constituents of Cannabis sativa, as well as endocannabinoids (eCBs). The eCBs are endogenously synthesized from the omega-6 and omega-3 polyunsaturated fatty acids (PUFAs). The consumption of omega-3 fatty acids shifts the balance towards a higher proportion of omega-3 eCBs, whose physiological functions warrants further investigation. Herein, we review the discovery of omega-3 fatty acid derived eCBs that are generated from long chain omega-3 PUFAs - docosahexaenoyl ethanolamide (DHA-EA or synaptamide), docosahexanoyl-glycerol (DHG), eicosapentaenoyl ethanolamide (EPA-EA) and eicosapentanoylglycerol (EPG). Furthermore, we outline the lesser known omega-3 eCB-like molecules that arise from the conjugation of omega-3 fatty acids with neurotransmitters serotonin and dopamine - DHA-serotonin (DHA-5HT), DHA-dopamine (DHA-DA), EPA-serotonin (EPA-5HT) and EPA-dopamine (EPA-DA). Additionally, we describe the role of omega-3 eCBs and their derivatives in different disease states, such as pain, inflammation and cancer. Moreover, we detail the formation and potential physiological roles of the oxidative metabolites that arise from the metabolism of omega-3 eCBs by eicosanoid synthesizing enzymes - cyclooxygenase (COX), lipoxygenase (LOX) and cytochrome P450 epoxygenase (CYP450). In summary, we outline the novel findings regarding a growing class of signaling molecules that can control the physiological and pathophysiological processes in the body.
Keywords: Cancer; Endocannabinoids; Inflammation; Metabolism; Omega-3 fatty acids.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest Statement
The authors declare no competing financial interest.
Figures
Figure 1.. Omega-3 endocannabinoids and derivatives.
Chemical Structures of (A) Omega-3 fatty acids, Docosahexaenoic Acid (DHA) and Eicosapentaenoic Acid (EPA) and N-acyl amide derivatives that have been identified in literature. (B) Docosahexaenoyl ethanolamide (DHA-EA), Eicosapentaenoyl ethanolamide (EPA-EA), (C) 1-Docosahexaenoyl-glycerol (1-DHG), 1-Eicosapentaenoyl- glycerol (1-EPG), (D) DHA-Dopamine (DHA-DA), EPA-Dopamine (EPA-DA), DHA-Serotonin (DHA-5HT) and EPA-Serotonin (EPA-5HT).
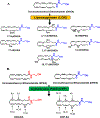
Figure 2.. Oxidation of Omega-3 endocannabinoids (DHA-EA and EPA-EA) by the LOX and CYP450 pathway.
(A) For the LOX pathway, oxidation can occur once and metabolites formed can undergo additionally oxidation or reduction to form seven DHA-EA-LOX metabolites; 17-hydroperoxydocosahexaenoyl ethanolamide (17-HpDHA-EA), 17-hydroxy-4Z,7Z,10Z,13Z,15E,19Z-docosahexaenoylethanolamide (17-HDHA-EA), 10,17-dihydroxydocosahexaenoyl ethanolamide (10,17-diHDHA-EA), 7,17-dihydroxydocosahexaenoyl ethanolamide (7,17-diHDHA-EA), 4,17-dihydroxydocosahexaenoyl ethanolamide (4,17-diHDHA-EA), 13-hydroxy-16(17)-epoxy-docosapentaenyolethanolamide (13-HEDPEA) and 15-hydroxy-16(17)-epoxy-docosapentaenyolethanolamide (15-HEDPEA). (B) The CYP450 pathway oxidizes DHA-EA and EPA-EA into six epoxydocosapentaenoic acid-ethanolamide (EDP-EA) and five epoxyeicosatetraenoic acid-ethanolamide (EEQ-EA) regioisomers, respectively.
Similar articles
- Anti-inflammatory ω-3 endocannabinoid epoxides.
McDougle DR, Watson JE, Abdeen AA, Adili R, Caputo MP, Krapf JE, Johnson RW, Kilian KA, Holinstat M, Das A. McDougle DR, et al. Proc Natl Acad Sci U S A. 2017 Jul 25;114(30):E6034-E6043. doi: 10.1073/pnas.1610325114. Epub 2017 Jul 7. Proc Natl Acad Sci U S A. 2017. PMID: 28687674 Free PMC article. - Dietary PUFAs and Exercise Dynamic Actions on Endocannabinoids in Brain: Consequences for Neural Plasticity and Neuroinflammation.
Park Y, Watkins BA. Park Y, et al. Adv Nutr. 2022 Oct 2;13(5):1989-2001. doi: 10.1093/advances/nmac064. Adv Nutr. 2022. PMID: 35675221 Free PMC article. Review. - Disentangling the Molecular Mechanisms of the Antidepressant Activity of Omega-3 Polyunsaturated Fatty Acid: A Comprehensive Review of the Literature.
Kalkman HO, Hersberger M, Walitza S, Berger GE. Kalkman HO, et al. Int J Mol Sci. 2021 Apr 22;22(9):4393. doi: 10.3390/ijms22094393. Int J Mol Sci. 2021. PMID: 33922396 Free PMC article. Review. - N-Acyl amines of docosahexaenoic acid and other n-3 polyunsatured fatty acids - from fishy endocannabinoids to potential leads.
Meijerink J, Balvers M, Witkamp R. Meijerink J, et al. Br J Pharmacol. 2013 Jun;169(4):772-83. doi: 10.1111/bph.12030. Br J Pharmacol. 2013. PMID: 23088259 Free PMC article. Review. - [Essential fatty acids and lipid mediators. Endocannabinoids].
Caramia G. Caramia G. Pediatr Med Chir. 2012 Mar-Apr;34(2):65-72. doi: 10.4081/pmc.2012.2. Pediatr Med Chir. 2012. PMID: 22730630 Italian.
Cited by
- Investigating the alterations of endocannabinoidome signaling in the human small intestine in the context of obesity and type 2 diabetes.
Rakotoarivelo V, Allam-Ndoul B, Martin C, Biertho L, Di Marzo V, Flamand N, Veilleux A. Rakotoarivelo V, et al. Heliyon. 2024 Mar 2;10(6):e26968. doi: 10.1016/j.heliyon.2024.e26968. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38515705 Free PMC article. - Lifestyle and Metabolic Syndrome: Contribution of the Endocannabinoidome.
Di Marzo V, Silvestri C. Di Marzo V, et al. Nutrients. 2019 Aug 20;11(8):1956. doi: 10.3390/nu11081956. Nutrients. 2019. PMID: 31434293 Free PMC article. Review. - Acute and long-term exercise differently modulate plasma levels of oxylipins, endocannabinoids, and their analogues in young sedentary adults: A sub-study and secondary analyses from the ACTIBATE randomized controlled-trial.
Jurado-Fasoli L, Di X, Sanchez-Delgado G, Yang W, Osuna-Prieto FJ, Ortiz-Alvarez L, Krekels E, Harms AC, Hankemeier T, Schönke M, Aguilera CM, Llamas-Elvira JM, Kohler I, Rensen PCN, Ruiz JR, Martinez-Tellez B. Jurado-Fasoli L, et al. EBioMedicine. 2022 Nov;85:104313. doi: 10.1016/j.ebiom.2022.104313. Epub 2022 Oct 27. EBioMedicine. 2022. PMID: 36374769 Free PMC article. Clinical Trial. - Wonder or evil?: Multifaceted health hazards and health benefits of Cannabis sativa and its phytochemicals.
Datta S, Ramamurthy PC, Anand U, Singh S, Singh A, Dhanjal DS, Dhaka V, Kumar S, Kapoor D, Nandy S, Kumar M, Koshy EP, Dey A, Proćków J, Singh J. Datta S, et al. Saudi J Biol Sci. 2021 Dec;28(12):7290-7313. doi: 10.1016/j.sjbs.2021.08.036. Epub 2021 Aug 19. Saudi J Biol Sci. 2021. PMID: 34867033 Free PMC article. Review. - Exploring the Role of Nutraceuticals in Major Depressive Disorder (MDD): Rationale, State of the Art and Future Prospects.
Alvarez-Mon MA, Ortega MA, García-Montero C, Fraile-Martinez O, Monserrat J, Lahera G, Mora F, Rodriguez-Quiroga A, Fernandez-Rojo S, Quintero J, Alvarez-Mon M. Alvarez-Mon MA, et al. Pharmaceuticals (Basel). 2021 Aug 21;14(8):821. doi: 10.3390/ph14080821. Pharmaceuticals (Basel). 2021. PMID: 34451918 Free PMC article. Review.
References
- LOEWE S [Active principals of the cannabis and the pharmacology of the cannabinols]. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1950;211(2):175–93. - PubMed
- Howlett AC. Inhibition of neuroblastoma adenylate cyclase by cannabinoid and nantradol compounds. Life Sci. 1984;35(17):1803–10. - PubMed
- Howlett AC. Cannabinoid inhibition of adenylate cyclase. Biochemistry of the response in neuroblastoma cell membranes. Mol Pharmacol. 1985;27(4):429–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials