The Canadian childhood nephrotic syndrome (CHILDNEPH) study: report on mid-study feasibility, recruitment and main measures - PubMed (original) (raw)
Observational Study
The Canadian childhood nephrotic syndrome (CHILDNEPH) study: report on mid-study feasibility, recruitment and main measures
Susan M Samuel et al. BMC Nephrol. 2019.
Abstract
Background: To assess reasons for continuing practice variation in the management of childhood nephrotic syndrome despite expert reviews and guidelines, we are conducting a longitudinal cohort study in children with glucocorticoid sensitive nephrotic syndrome. Objectives of this mid-study report are to describe patient and physician recruitment characteristics, glucocorticoid prescriptions, use of second line agents, biopsy practices, and adherence to study protocol.
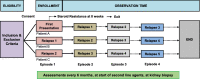
Methods: Children with new onset nephrotic syndrome and providers are being recruited from all 12 pediatric nephrology centres across Canada with > 2½ years follow-up. Data collection points of observation are over a minimum 36 months. Details of prescribed glucocorticoids and of all second line agents used during treatment are being collected. All relapses are being recorded with time to urinary remission of proteinuria.
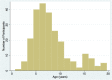
Results: To date, 243 patients (57.1% male) from 12 centres were included. Median number of patients per centre was 29 (range 2-45), and median age of cohort was 7.3 (IQR 4.2) at enrollment. Forty-eight physicians were recruited, median 5 (range 2-8) per site. Median number of relapses per patient year of follow-up was 2.1 (IQR 4). Cumulative dose variability of glucocorticoids prescribed per episode of proteinuria and length of treatment was observed between participating centres.
Conclusion: The Canadian pediatric nephrology community established a longitudinal childhood nephrotic syndrome cohort study that confirms ongoing practice variability. The study will help to evaluate its impact on patient outcomes, and facilitate clinical trial implementation in nephrotic syndrome.
Keywords: Children; Glucocorticoids; Longitudinal study; Nephrotic syndrome; Practice variation.
Conflict of interest statement
Ethics approval and consent to participate
Ethics approval was obtained from the University of Calgary (REB13–0059) in 2013, and subsequently, the study was approved by research ethics boards at all participating sites. Written parental/guardian consent and patient assent as applicable, were obtained prior to initiating study activities. Consent was also obtained from physicians before their participation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests with respect to this manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Fig. 1
Data collection time points during observation
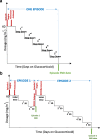
Fig. 2
Defining an episode, illustrating sample scenarios. An episode is defined as the time from start of full dose glucocorticoid therapy (60 mg/m2 or 2 mg/kg) to cessation of glucocorticoids (a) or re-start of full dose glucocorticoids as in glucocorticoid dependent patients who relapse while tapering glucocorticoids. (b) Note that the glucocorticoid tapering mode may vary between prescribers. Abbreviation: SD – step down
Fig. 3
Distribution of age at enrollment into the study
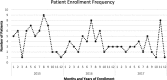
Fig. 4
Seasonal variation in enrollment and all episodes recorded
Fig. 5
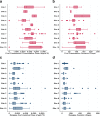
Site specific differences of cumulative dose of glucocorticoid given (a) and the length of treatment (b) at first presentation. Further illustrated are the site-specific differences of cumulative dose of glucocorticoid given (c) and length of treatment (d) at relapse. Data from 2 sites were not shown due to low enrollment at the time of this report. Note: I Sites 1, 3,5,6,7,9,10 used protocol based treatments for nephrotic syndrome while Sites 2, 4, 8 did not. II Number of patients for sites 1–10 in first presentation graphs were 45, 3, 2, 37, 8, 29, 30, 22, 36 and 29 respectively. III Number of patients for sites 1–10 in relapse graphs were 32, 2, 1, 22, 6, 20, 22, 17, 24 and 18 respectively
Similar articles
- Characteristics Associated with Variation in Corticosteroid Exposure in Children with Steroid-Sensitive Nephrotic Syndrome: Results from a Canadian Longitudinal Study.
Rodriguez-Lopez S, Chanchlani R, Dart AB, Morgan CJ, Lapeyraque AL, Tee JB, Brobbey A, Perinpanayagam MA, Samuel S, Nettel-Aguirre A. Rodriguez-Lopez S, et al. Kidney360. 2021 Sep 23;2(12):1960-1967. doi: 10.34067/KID.0002692021. eCollection 2021 Dec 30. Kidney360. 2021. PMID: 35419527 Free PMC article. - IPNA clinical practice recommendations for the diagnosis and management of children with steroid-sensitive nephrotic syndrome.
Trautmann A, Boyer O, Hodson E, Bagga A, Gipson DS, Samuel S, Wetzels J, Alhasan K, Banerjee S, Bhimma R, Bonilla-Felix M, Cano F, Christian M, Hahn D, Kang HG, Nakanishi K, Safouh H, Trachtman H, Xu H, Cook W, Vivarelli M, Haffner D; International Pediatric Nephrology Association. Trautmann A, et al. Pediatr Nephrol. 2023 Mar;38(3):877-919. doi: 10.1007/s00467-022-05739-3. Epub 2022 Oct 21. Pediatr Nephrol. 2023. PMID: 36269406 Free PMC article. Review. - [Multicenter survey of diagnostic and therapeutic status in Chinese childhood patients with steroid-sensitive, relaping/steroid-dependent nephrotic syndrome].
Working Group For National Survey On Status Of Diagnosis And Treatment Of Childhood Renal Diseases. Working Group For National Survey On Status Of Diagnosis And Treatment Of Childhood Renal Diseases. Zhonghua Er Ke Za Zhi. 2014 Mar;52(3):194-200. Zhonghua Er Ke Za Zhi. 2014. PMID: 24824389 Chinese. - Canadian Society of Nephrology Commentary on the 2012 KDIGO clinical practice guideline for glomerulonephritis: management of nephrotic syndrome in children.
Samuel S, Bitzan M, Zappitelli M, Dart A, Mammen C, Pinsk M, Cybulsky AV, Walsh M, Knoll G, Hladunewich M, Bargman J, Reich H, Humar A, Muirhead N. Samuel S, et al. Am J Kidney Dis. 2014 Mar;63(3):354-62. doi: 10.1053/j.ajkd.2013.12.002. Epub 2014 Jan 11. Am J Kidney Dis. 2014. PMID: 24423782 Review. - Induction prednisone dosing for childhood nephrotic syndrome: how low should we go?
Sibley M, Roshan A, Alshami A, Catapang M, Jöbsis JJ, Kwok T, Polderman N, Sibley J, Matsell DG, Mammen C; Pediatric Nephrology Clinical Pathway Development Team. Sibley M, et al. Pediatr Nephrol. 2018 Sep;33(9):1539-1545. doi: 10.1007/s00467-018-3975-6. Epub 2018 May 22. Pediatr Nephrol. 2018. PMID: 29789934
Cited by
- The urgent need for conducting clinical trials in pediatric nephrology globally.
Wightman A, Filler G, Díaz-González de Ferris ME. Wightman A, et al. Pediatr Nephrol. 2023 Aug;38(8):2499-2506. doi: 10.1007/s00467-023-05877-2. Epub 2023 Feb 4. Pediatr Nephrol. 2023. PMID: 36738331 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical