Mechanistic Insights into the Antimicrobial Actions of Metallic Nanoparticles and Their Implications for Multidrug Resistance - PubMed (original) (raw)
Review
Mechanistic Insights into the Antimicrobial Actions of Metallic Nanoparticles and Their Implications for Multidrug Resistance
Sibhghatulla Shaikh et al. Int J Mol Sci. 2019.
Abstract
Multiple drug-resistant bacteria are a severe and growing public health concern. Because relatively few antibiotics have been approved over recent years and because of the inability of existing antibiotics to combat bacterial infections fully, demand for unconventional biocides is intense. Metallic nanoparticles (NPs) offer a novel potential means of fighting bacteria. Although metallic NPs exert their effects through membrane protein damage, superoxide radicals and the generation of ions that interfere with the cell granules leading to the formation of condensed particles, their antimicrobial potential, and mechanisms of action are still debated. This article discusses the action of metallic NPs as antibacterial agents, their mechanism of action, and their effect on bacterial drug resistance. Based on encouraging data about the antibacterial effects of NP/antibiotic combinations, we propose that this concept be thoroughly researched to identify means of combating drug-resistant bacteria.
Keywords: antimicrobials agents; drug resistance; nanomaterials; physico-chemical property; superoxide radicals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
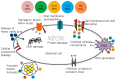
Figure 1
Schematic representations of the antimicrobial mechanisms of various nanoparticles (NPs).
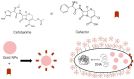
Figure 2
Proposed antibacterial mechanisms of antibiotic-conjugated AuNPs. AuNPs act as antibiotic carriers and facilitate access to bacterial cell walls. Cefaclor or cefotaxime damage cell walls and enable AuNP entry, and AuNPs then prevent DNA from unwinding.
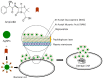
Figure 3
Proposed antibacterial mechanisms of ampicillin plus AgNPs. AgNPs-ampicillin in combination hinders the formation of crosslinks in the peptidoglycan layer, which results in cell lysis. AgNPs-ampicillin complex inhibits DNA unwinding.
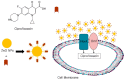
Figure 4
Schematic representation of the antibacterial mechanism of ciprofloxacin conjugated ZnO-NPs. ZnO-NP-Ciprofloxacin complex interferes with NorA (endogenous efflux transporter) and membrane Omf proteins, and thus enhances ciprofloxacin entry.
Similar articles
- Exploring the potential of metal and metal oxide nanomaterials for sustainable water and wastewater treatment: A review of their antimicrobial properties.
Kamyab H, Chelliapan S, Hayder G, Yusuf M, Taheri MM, Rezania S, Hasan M, Yadav KK, Khorami M, Farajnezhad M, Nouri J. Kamyab H, et al. Chemosphere. 2023 Sep;335:139103. doi: 10.1016/j.chemosphere.2023.139103. Epub 2023 Jun 2. Chemosphere. 2023. PMID: 37271472 Review. - Metallic Nanoparticles: A Promising Arsenal against Antimicrobial Resistance-Unraveling Mechanisms and Enhancing Medication Efficacy.
Wahab S, Salman A, Khan Z, Khan S, Krishnaraj C, Yun SI. Wahab S, et al. Int J Mol Sci. 2023 Oct 4;24(19):14897. doi: 10.3390/ijms241914897. Int J Mol Sci. 2023. PMID: 37834344 Free PMC article. Review. - Metallic nanoparticles induced antibiotic resistance genes attenuation of leachate culturable microbiota: The combined roles of growth inhibition, ion dissolution and oxidative stress.
Su Y, Wu D, Xia H, Zhang C, Shi J, Wilkinson KJ, Xie B. Su Y, et al. Environ Int. 2019 Jul;128:407-416. doi: 10.1016/j.envint.2019.05.007. Epub 2019 May 9. Environ Int. 2019. PMID: 31078875 - The Role of Nanoparticles in the Inhibition of Multidrug-Resistant Bacteria and Biofilms.
AlMatar M, Makky EA, Var I, Koksal F. AlMatar M, et al. Curr Drug Deliv. 2018;15(4):470-484. doi: 10.2174/1567201815666171207163504. Curr Drug Deliv. 2018. PMID: 29219055 Review. - Essential Oils and Mono/bi/tri-Metallic Nanocomposites as Alternative Sources of Antimicrobial Agents to Combat Multidrug-Resistant Pathogenic Microorganisms: An Overview.
Basavegowda N, Patra JK, Baek KH. Basavegowda N, et al. Molecules. 2020 Feb 27;25(5):1058. doi: 10.3390/molecules25051058. Molecules. 2020. PMID: 32120930 Free PMC article. Review.
Cited by
- Quorum Quenching Approaches against Bacterial-Biofilm-Induced Antibiotic Resistance.
D'Aquila P, De Rose E, Sena G, Scorza A, Cretella B, Passarino G, Bellizzi D. D'Aquila P, et al. Antibiotics (Basel). 2024 Jul 3;13(7):619. doi: 10.3390/antibiotics13070619. Antibiotics (Basel). 2024. PMID: 39061301 Free PMC article. Review. - Photocatalytic and Antibacterial Properties of Doped TiO2 Nanopowders Synthesized by Sol-Gel Method.
Preda S, Pandele-Cușu J, Petrescu SV, Ciobanu EM, Petcu G, Culiță DC, Apostol NG, Costescu RM, Raut I, Constantin M, Predoană L. Preda S, et al. Gels. 2022 Oct 20;8(10):673. doi: 10.3390/gels8100673. Gels. 2022. PMID: 36286174 Free PMC article. - Gold Nanoparticle-Based Resuscitation of Cefoxitin against Clinical Pathogens: A Nano-Antibiotic Strategy to Overcome Resistance.
Alafnan A, Rizvi SMD, Alshammari AS, Faiyaz SSM, Lila ASA, Katamesh AA, Khafagy ES, Alotaibi HF, Ahmed ABF. Alafnan A, et al. Nanomaterials (Basel). 2022 Oct 18;12(20):3643. doi: 10.3390/nano12203643. Nanomaterials (Basel). 2022. PMID: 36296833 Free PMC article. - Metallic Structures: Effective Agents to Fight Pathogenic Microorganisms.
Pereira D, Carreira TS, Alves N, Sousa Â, Valente JFA. Pereira D, et al. Int J Mol Sci. 2022 Jan 21;23(3):1165. doi: 10.3390/ijms23031165. Int J Mol Sci. 2022. PMID: 35163090 Free PMC article. Review.
References
- Qureshi M., Asif N., Baig S. Evaluation of extended spectrum β-lactamase mediated resistance in Escherichia coli and Klebsiella in urinary tract infection at a tertiary care hospital. Biomedica. 2013;29:78–81.
- Shaikh S., Rizvi S.M.D., Shakil S., Ahmad A., Pathak N. Non-clonal dissemination of extended-spectrum beta-lactamase-producing pseudomonas aeruginosa strains of clinical origin. Iran. J. Sci. Technol. Trans. A Sci. 2017;41:1011–1015. doi: 10.1007/s40995-017-0340-8. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous