Functional cooperation of α-synuclein and VAMP2 in synaptic vesicle recycling - PubMed (original) (raw)
Functional cooperation of α-synuclein and VAMP2 in synaptic vesicle recycling
Jichao Sun et al. Proc Natl Acad Sci U S A. 2019.
Abstract
The function of α-synuclein (α-syn) has been long debated, and two seemingly divergent views have emerged. In one, α-syn binds to VAMP2, acting as a SNARE chaperone-but with no effect on neurotransmission-while another posits that α-syn attenuates neurotransmitter release by restricting synaptic vesicle mobilization and recycling. Here, we show that α-syn-VAMP2 interactions are necessary for α-syn-induced synaptic attenuation. Our data connect divergent views and suggest a unified model of α-syn function.
Keywords: Parkinson’s disease; alpha synuclein; synaptic transmission.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
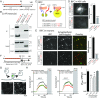
Fig. 1.
α-Syn sequence lacking the VAMP2-binding domain fails to attenuate SV recycling. (A and B) α-Syn sequence (A) and co-IP of α-syn–VAMP2 (B). Neuro2a cells were cotransfected with myc-tagged α-syn and VAMP2 and then immunoprecipitated with an anti-myc antibody. Note that α-syn 96–140 is the VAMP2-binding region (repeated twice). (C) Principle of our BiFC assay. (D) HEK cells were transfected with VN:VAMP2 and α-syn:VC (α-syn 1–140 or 1–95). Note punctate fluorescence with VN:VAMP2 + α-syn(1–140):VC, which was greatly attenuated with α-syn 1–95 sequence lacking VAMP2-binding domain; quantification of data shown Right (mean ± SEM; α-syn 1–140, 1 ± 0.06499, n = 38 from 3 independent experiments; α-syn 1–95, 0.3074 ± 0.01676, n = 39; ****P < 0.0001). (E) Cultured hippocampal neurons were cotransfected with the constructs listed; note α-syn–VAMP2 Venus complementation at boutons with WT α-syn; quantification shown Right (mean ± SEM from 2 independent experiments; α-syn 1–140, 1 ± 0.03949, n = 280; α-syn 1–95, 0.1691 ± 0.007379, n = 312; ****P < 0.0001). (F) α-Syn 1–110 contains the VAMP2-binding site that starts at amino acid 96. (G) Principle of pHluorin assay (Top) with representative images (Below). (H and I) Experiments in cultured hippocampal neurons (pHluorin). (H) While α-syn 1–110 attenuates SV recycling (Left), α-syn 1–95 has no effect (Right) (note that some error bars are too small to see). (I) Quantification of data in H (mean ± SEM from at least 3 independent experiments); control, 0.4703 ± 0.03215, n = 16; α-syn 1–140, 0.3165 ± 0.02914, n = 13; α-syn 1–110, 0.3464 ± 0.05906, n = 8; α-syn 1–95, 0.4763 ± 0.0192, n = 17; **P = 0.0025, *P = 0.0459 (one-way ANOVA followed by Dunnett’s post hoc test).
Fig. 2.
Mapping of the α-syn–VAMP2 binding domain and requirement of α-syn–VAMP2 interactions for α-syn–mediated SV attenuation. (A and B) Both α-syn 1–140 and 1–110 bind VAMP2 with equal affinity, and scrambling of amino acid sequences in α-syn 96–110 attenuates α-syn–VAMP2 binding (co-IP in neuro2a, repeated twice). (C and D) Sequential amino acids (from α-syn 96) were mutated to alanine, and association of these mutants (myc-tagged) with VAMP2 was evaluated (co-IP in neuro2a cells). Note that mutations in α-syn 96–104 show the greatest disruption (repeated twice). (E) Scrambled and KKD mutations in the α-syn 96–110 sequence abrogated α-syn–mediated synaptic attenuation, as determined by pHluorin assays in hippocampal neurons. (F) Quantification of data in E (mean ± SEM from at least 3 independent experiments); control, 0.423 ± 0.029, n = 6; α-syn 1–140, 0.178 ± 0.032, n = 6; KKD, 0.453 ± 0.070, n = 5; Scr-1, 0.464 ± 0.041, n = 6; **P = 0.0017 (one-way ANOVA followed by Dunnett’s post hoc test). (G_–_I) Optical single vesicle clustering experiments were carried out as described in ref. . Briefly, VAMP2-containing synaptic-like vesicles were first immobilized on a glass slide assembled in a microfluidic chamber, and then WT or mutant α-syn protein was added. After extensive washing (to remove unbound α-syn), DiI-labeled VAMP2-containing vesicles were added to the chamber, and clustering of the labeled vesicles was visualized by prism-type total internal reflection fluorescence microscopy (after extensive washing to remove unbound vesicles). As shown in representative images (G) and quantitative data (H), α-syn induced vesicle clustering, and deletions or subtle mutations in the VAMP2-binding site markedly abrogated the number of vesicle clusters. Mean ± SEM from 4 independent experiments where observer was blinded to the conditions; α-syn 1–140, 100%; α-syn 1–110, 83.15% ± 6.439%; no α-syn, 21.83% ± 7.437%; α-syn 1–95, 29.94% ± 8.332%; KKD, 33.89% ± 3.465%; Scr-1, 30.49% ± 8.138%; NS, nonsignificant; ****P < 0.001 (one-way ANOVA followed by Dunnett’s post hoc test). (I) Scatter plots showing number of vesicle clusters (on y axis) and fluorescence intensities (on x axis) of all Dil-labeled clusters, along with a smoothened curve through the data points.
Similar articles
- α-Synuclein may cross-bridge v-SNARE and acidic phospholipids to facilitate SNARE-dependent vesicle docking.
Lou X, Kim J, Hawk BJ, Shin YK. Lou X, et al. Biochem J. 2017 Jun 6;474(12):2039-2049. doi: 10.1042/BCJ20170200. Biochem J. 2017. PMID: 28495859 Free PMC article. - Functional and Pathological Effects of α-Synuclein on Synaptic SNARE Complexes.
Gao V, Briano JA, Komer LE, Burré J. Gao V, et al. J Mol Biol. 2023 Jan 15;435(1):167714. doi: 10.1016/j.jmb.2022.167714. Epub 2022 Jul 3. J Mol Biol. 2023. PMID: 35787839 Free PMC article. Review. - The Role of α-Synuclein in SNARE-mediated Synaptic Vesicle Fusion.
Yoo G, Shin YK, Lee NK. Yoo G, et al. J Mol Biol. 2023 Jan 15;435(1):167775. doi: 10.1016/j.jmb.2022.167775. Epub 2022 Aug 3. J Mol Biol. 2023. PMID: 35931109 Review. - Nonaggregated α-synuclein influences SNARE-dependent vesicle docking via membrane binding.
Lai Y, Kim S, Varkey J, Lou X, Song JK, Diao J, Langen R, Shin YK. Lai Y, et al. Biochemistry. 2014 Jun 24;53(24):3889-96. doi: 10.1021/bi5002536. Epub 2014 Jun 13. Biochemistry. 2014. PMID: 24884175 Free PMC article. - α-synuclein multimers cluster synaptic vesicles and attenuate recycling.
Wang L, Das U, Scott DA, Tang Y, McLean PJ, Roy S. Wang L, et al. Curr Biol. 2014 Oct 6;24(19):2319-26. doi: 10.1016/j.cub.2014.08.027. Epub 2014 Sep 25. Curr Biol. 2014. PMID: 25264250 Free PMC article.
Cited by
- Oligomerization of Lrrk controls actin severing and α-synuclein neurotoxicity in vivo.
Sarkar S, Bardai F, Olsen AL, Lohr KM, Zhang YY, Feany MB. Sarkar S, et al. Mol Neurodegener. 2021 May 24;16(1):33. doi: 10.1186/s13024-021-00454-3. Mol Neurodegener. 2021. PMID: 34030727 Free PMC article. - Alpha-Synuclein Aggregation Pathway in Parkinson's Disease: Current Status and Novel Therapeutic Approaches.
Vidović M, Rikalovic MG. Vidović M, et al. Cells. 2022 May 24;11(11):1732. doi: 10.3390/cells11111732. Cells. 2022. PMID: 35681426 Free PMC article. Review. - T cells, α-synuclein and Parkinson disease.
Garretti F, Monahan C, Sette A, Agalliu D, Sulzer D. Garretti F, et al. Handb Clin Neurol. 2022;184:439-455. doi: 10.1016/B978-0-12-819410-2.00023-0. Handb Clin Neurol. 2022. PMID: 35034753 Free PMC article. Review. - α-Synuclein Disrupts Vesicle Fusion by Two Mutant-Specific Mechanisms.
Yoo G, An HJ, Yeou S, Lee NK. Yoo G, et al. Mol Cells. 2022 Nov 30;45(11):806-819. doi: 10.14348/molcells.2022.0102. Epub 2022 Nov 11. Mol Cells. 2022. PMID: 36380732 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous