Human Hippocampal Neurogenesis Persists in Aged Adults and Alzheimer's Disease Patients - PubMed (original) (raw)
Human Hippocampal Neurogenesis Persists in Aged Adults and Alzheimer's Disease Patients
Matthew K Tobin et al. Cell Stem Cell. 2019.
Abstract
Whether hippocampal neurogenesis persists throughout life in the human brain is not fully resolved. Here, we demonstrate that hippocampal neurogenesis is persistent through the tenth decade of life and is detectable in patients with mild cognitive impairments and Alzheimer's disease. In a cohort of 18 participants with a mean age of 90.6 years, Nestin+Sox2+ neural progenitor cells (NPCs) and DCX+ neuroblasts and immature neurons were detected, but their numbers greatly varied between participants. Nestin+ cells localize in the anterior hippocampus, and NPCs, neuroblasts, and immature neurons are evenly distributed along the anterior to posterior axis. The number of DCX+PCNA+ cells is reduced in mild cognitive impairments, and higher numbers of neuroblasts are associated with better cognitive status. The number of DCX+PCNA+ cells correlates with functional interactions between presynaptic SNARE proteins. Our results suggest that hippocampal neurogenesis persists in the aged and diseased human brain and that it is possibly associated with cognition.
Keywords: Alzheimer’s disease; adult neurogenesis; aging; cognitive dysfunction; human neurogenesis; neural stem cells; neurogenesis in aging.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests: The authors declare no competing financial interests.
Figures
Figure 1.. Counts of neural progenitor cells in the hippocampus of aged humans.
a-c. Cell counts in the SGL: Nestin+ (a), PCNA+ (b), Nestin+PCNA+ (c). d-f. Cell counts in the GCL: Nestin+ (d), PCNA+ (e), and Nestin+PCNA+ (f). g-i. Cell counts upweighted to DG volume: Nestin+ (g), PCNA+ (h), and Nestin+PCNA+ (i). j-l. Total cell counts in the DG: Nestin+Sox2+ (j), Nestin+Ki67+ (k), Nestin+Sox2+Ki67+ (l). m-o Cell counts upweighted to DG volume. Nestin+ (m), Nestin+Ki67+ (n), Nestin+Sox2+Ki67+ (o). Data represent the mean ± SEM of all 18 subject with each data point corresponding to a single subject. Clinical diagnosis is color coded (black = normal, no cognitive impairments, blue = mild cognitive impairments, red = Alzheimer’s disease). p,q. Representative images of Nestin+Sox2+ (p) or Nestin+Sox2+Ki67+ (q) -expressing cells. (GCL and SGL are noted, white outline = area presented in high power on the right). Scale bar represents low magnifications images (left panel): 20 μm, high magnification images (right panel): 10 μm.
Figure 2.. Quantification of neuroblasts and immature neurons in the hippocampus of aged humans.
a-c. Number of DCX+ cells in the SGL (a), GCL (b) and whole DG (c). d-f. Number of DCX+PCNA+ cells in the SGL (d), GCL (e) and whole DG (f). Data represent the mean ± SEM of all 18 subject with each data point corresponding to a single subject. Clinical diagnosis is color coded (black = normal, no cognitive impairments, blue = mild cognitive impairments, red = Alzheimer’s disease). g. Representative images of DCX - expressing cells co-stained with DAPI (GCL and SGL are noted, white outline = area presented in high power on the right). Scale bar Top and Middle Panels: low magnification 20 μm, high magnification 20 μm. Bottom Panel: low magnification 10 μm, high magnification 20 μm.
Figure 3.. Correlation of cell numbers along the dorsal/ventral axis of the hippocampus.
a-c. Correlation of Nestin+ (a), PCNA+ (b), and DCX+ (c) cell counts in the SGL. d-f. Correlation of Nestin+ (d), PCNA+ (e), and DCX+ (f) cell counts in the GCL. g-n. Correlation of total Nestin+ (g), PCNA+ (h), DCX+ (i), Nestin+Sox2+ (j), Nestin+PCNA+ (k), Nestin+Ki67+ (l), Nestin+Sox2+Ki67+ (m) and DCX+PCNA+ (n) cell counts upweighted to DG volume. Coefficients (β) were estimated with each cell volume normalized with mean and standard deviation.
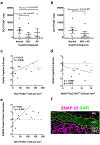
Figure 4.. Association of neurogenesis with cognitive diagnosis and presynaptic proteins.
a,b. Logistic regression analysis of cognitive diagnosis and the number of DCX+PCNA+ cells shows significant correlation with MCI (p=0.038) (a) and borderline significance with MCI+AD (p=0.067) (b). c,d. Association between the number of DCX+PCNA+ (c) or Nestin+Sox2+Ki67+ (d) cells and global cognition score (p=0.046, p=0.098, respectively). e. Association between the number of DCX+PCNA+ cells and SNARE protein-protein interaction (p=0.0038). f. Representative image showing the distribution of presynaptic proteins in the dentate gyrus of the hippocampus inner molecular layer (IML), granule cell layer (GCL), subgranular layer (SGCL) and CA4 subfield. SNAP-25 immunostaining (magenta) with cell nuclei stained with DAPI (green) in a section of brain where DCX+PCNA+ cells were identified. Scale bar representes 25 μm.
Comment in
- Is Alzheimer's Disease a Neurogenesis Disorder?
Choi SH, Tanzi RE. Choi SH, et al. Cell Stem Cell. 2019 Jul 3;25(1):7-8. doi: 10.1016/j.stem.2019.06.001. Cell Stem Cell. 2019. PMID: 31271749
Similar articles
- Early Changes in Hippocampal Neurogenesis in Transgenic Mouse Models for Alzheimer's Disease.
Unger MS, Marschallinger J, Kaindl J, Höfling C, Rossner S, Heneka MT, Van der Linden A, Aigner L. Unger MS, et al. Mol Neurobiol. 2016 Oct;53(8):5796-806. doi: 10.1007/s12035-016-0018-9. Epub 2016 Aug 20. Mol Neurobiol. 2016. PMID: 27544234 Free PMC article. - FGF Signaling Is Necessary for Neurogenesis in Young Mice and Sufficient to Reverse Its Decline in Old Mice.
Kang W, Hébert JM. Kang W, et al. J Neurosci. 2015 Jul 15;35(28):10217-23. doi: 10.1523/JNEUROSCI.1469-15.2015. J Neurosci. 2015. PMID: 26180198 Free PMC article. - Influence of mitochondrial enzyme deficiency on adult neurogenesis in mouse models of neurodegenerative diseases.
Calingasan NY, Ho DJ, Wille EJ, Campagna MV, Ruan J, Dumont M, Yang L, Shi Q, Gibson GE, Beal MF. Calingasan NY, et al. Neuroscience. 2008 Jun 2;153(4):986-96. doi: 10.1016/j.neuroscience.2008.02.071. Epub 2008 Mar 18. Neuroscience. 2008. PMID: 18423880 Free PMC article. - Therapeutic modulation of neurogenesis to improve hippocampal plasticity and cognition in aging and Alzheimer's disease.
Mostafa M, Disouky A, Lazarov O. Mostafa M, et al. Neurotherapeutics. 2025 Apr;22(3):e00580. doi: 10.1016/j.neurot.2025.e00580. Epub 2025 Apr 2. Neurotherapeutics. 2025. PMID: 40180804 Free PMC article. Review. - Adult Hippocampal Neurogenesis in Aging and Alzheimer's Disease.
Babcock KR, Page JS, Fallon JR, Webb AE. Babcock KR, et al. Stem Cell Reports. 2021 Apr 13;16(4):681-693. doi: 10.1016/j.stemcr.2021.01.019. Epub 2021 Feb 25. Stem Cell Reports. 2021. PMID: 33636114 Free PMC article. Review.
Cited by
- Dietary Flavonoids and Adult Neurogenesis: Potential Implications for Brain Aging.
Davinelli S, Medoro A, Ali S, Passarella D, Intrieri M, Scapagnini G. Davinelli S, et al. Curr Neuropharmacol. 2023;21(3):651-668. doi: 10.2174/1570159X21666221031103909. Curr Neuropharmacol. 2023. PMID: 36321225 Free PMC article. Review. - The Effects of Dietary Intervention and Macrophage-Activating Factor Supplementation on Cognitive Function in Elderly Users of Outpatient Rehabilitation.
Uchiyama-Tanaka Y, Yamakage H, Inui T. Uchiyama-Tanaka Y, et al. Nutrients. 2024 Jun 28;16(13):2078. doi: 10.3390/nu16132078. Nutrients. 2024. PMID: 38999825 Free PMC article. Clinical Trial. - Electroconvulsive therapy is associated with increased immunoreactivity of neuroplasticity markers in the hippocampus of depressed patients.
Loef D, Tendolkar I, van Eijndhoven PFP, Hoozemans JJM, Oudega ML, Rozemuller AJM, Lucassen PJ, Dols A, Dijkstra AA. Loef D, et al. Transl Psychiatry. 2023 Nov 20;13(1):355. doi: 10.1038/s41398-023-02658-1. Transl Psychiatry. 2023. PMID: 37981649 Free PMC article. - [Development in Tissue Clearing Technology and Its Application in Neurodegenerative Diseases].
Gu PL, Shen JL, Zhu Y, Li J, Wang LH. Gu PL, et al. Sichuan Da Xue Xue Bao Yi Xue Ban. 2021 May;52(3):350-356. doi: 10.12182/20210560302. Sichuan Da Xue Xue Bao Yi Xue Ban. 2021. PMID: 34018350 Free PMC article. Review. Chinese. - Chronic Treatment with Melatonin Improves Hippocampal Neurogenesis in the Aged Brain and Under Neurodegeneration.
Cachán-Vega C, Vega-Naredo I, Potes Y, Bermejo-Millo JC, Rubio-González A, García-González C, Antuña E, Bermúdez M, Gutiérrez-Rodríguez J, Boga JA, Coto-Montes A, Caballero B. Cachán-Vega C, et al. Molecules. 2022 Aug 29;27(17):5543. doi: 10.3390/molecules27175543. Molecules. 2022. PMID: 36080336 Free PMC article.
References
- Aizawa K, Ageyama N, Terao K, and Hisatsune T (2011). Primate-specific alterations in neural stem/progenitor cells in the aged hippocampus. Neurobiol Aging 32, 140–150. - PubMed
- Bannerman DM, Grubb M, Deacon RM, Yee BK, Feldon J, and Rawlins JN (2003). Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res 139, 197–213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UH2 NS100599/NS/NINDS NIH HHS/United States
- R01 AG017917/AG/NIA NIH HHS/United States
- P30 CA060553/CA/NCI NIH HHS/United States
- P30 AG010161/AG/NIA NIH HHS/United States
- R01 AG062251/AG/NIA NIH HHS/United States
- R01 AG034374/AG/NIA NIH HHS/United States
- UF1 NS100599/NS/NINDS NIH HHS/United States
- R01 AG033570/AG/NIA NIH HHS/United States
- R01 AG060238/AG/NIA NIH HHS/United States
- RF1 AG033570/AG/NIA NIH HHS/United States
- R21 AG061628/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous