YAP/TAZ Signaling and Resistance to Cancer Therapy - PubMed (original) (raw)
Review
YAP/TAZ Signaling and Resistance to Cancer Therapy
Chan D K Nguyen et al. Trends Cancer. 2019 May.
Abstract
Drug resistance is a major challenge in cancer treatment. Emerging evidence indicates that deregulation of YAP/TAZ signaling may be a major mechanism of intrinsic and acquired resistance to various targeted and chemotherapies. Moreover, YAP/TAZ-mediated expression of PD-L1 and multiple cytokines is pivotal for tumor immune evasion. While direct inhibitors of YAP/TAZ are still under development, FDA-approved drugs that indirectly block YAP/TAZ activation or critical downstream targets of YAP/TAZ have shown promise in the clinic in reducing therapy resistance. Finally, BET inhibitors, which reportedly block YAP/TAZ-mediated transcription, present another potential venue to overcome YAP/TAZ-induced drug resistance.
Keywords: Cancer therapy resistance; Hippo pathway; TAZ; YAP.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures
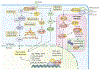
Figure 1.. YAP/TAZ signaling and resistance to targeted therapy.
The Hippo pathway, which consists of a core MST1/2-LATS1/2 kinase cassette and numerous regulatory proteins including NF2/Merlin, SAV1, MOB1, MOB3B, phosphorylates and inhibits YAP/TAZ by targeting them for cytoplasmic retention and proteasomal degradation mediated by 14-3-3 and β-TrCP, respectively. Inactivation of Hippo signaling promotes stabilization and nuclear entry of YAP/TAZ, where they form transcription complexes with TEAD1–4, AP-1, BRD4, ZEB1, E2F and possibly other transcription factors to induce the expression of pro-survival/anti-apoptotic genes such as CTGF, CYR61, Bcl-xL and Survivin. YAP/TAZ have been identified as major drivers of resistance to RAF and MEK inhibitors as single agents or in combination with TBK1 inhibitor in BRAF and KRAS mutant cancer cells. BRAFi treatment induces actin cytoskeletal remodeling and promotes Rho-mediated YAP nuclear entry in BRAF-mutant melanoma cells. YAP induces E2F and AP-1-mediated cell cycle and EMT transcriptional programs to promote survival following ablation of oncogenic Kras in pancreatic and colon cancer cells. Additionally, YAP/TAZ induce EGFRi resistance by increasing the expression of AXL, ERBB3 and EGFR.
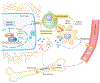
Figure 2.. YAP/TAZ signaling and resistance to chemotherapy.
Multiple miRNAs are up- or down-regulated in response to various chemo drugs and induce chemo resistance by targeting different components of the Hippo pathway (MST1, SAV1, LATS2, MOB1, YAP) in various cancer types. Anti-microtubule treatment activates CDK1, which phosphorylates and promotes LATS binding to and inhibition of Pin1, an oncoprotein that induces taxane resistance in multiple malignancies. Furthermore, CDK1 directly phosphorylates and inhibits YAP/TAZ by disrupting their binding to TEADs and destabilizing TAZ, sensitizing tumor cells to anti-microtubule drugs. The transcriptional activity of YAP is further modulated by CBP/p300-mediated acetylation and SIRT1-mediated deacetylation in response to various chemo agents. YAP/TAZ induce the transcription of antiapoptotic proteins (Bcl-xL, IAP1, Survivin.) and ABC multi-drug transporters to promote tumor cell survival and drug efflux, respectively. In addition, CTGF and CYR61, two canonical transcription targets of YAP/TAZ, can act through integrin receptors to activate MAPK and NF-κB signaling to further boost chemotherapy resistance.
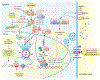
Figure 3.. YAP/TAZ signaling and resistance to immune checkpoint therapy.
YAP/TAZ/TEAD directly transcribe PD-L1 and multiple cytokines (CSF1–3, IL6, CXCL5). YAP/TAZ-mediated PD-L1 expression on tumor cells binds to PD-1 expressed on effector T cells (CTLs), leading to inactivation of CTLs. Furthermore, YAP-induced upregulation of IL-6 and CSF1–3 skew the monocyte polarization in favor of tumor-promoting MDSCs and TAMs in bone marrow. On the other hand, YAP-mediated expression of CXCL5 by tumor cells attracts MDSCs and TAMs through its cognate receptor CXCR2 expressed on MDSCs and TAMs. Once recruited into the tumor microenvironment, MDSCs and TAMs inactivate CTLs both directly via PD-L1-PD interaction and indirectly by upregulating Arg1 and various other mechanisms.
Similar articles
- Multiple roles and context-specific mechanisms underlying YAP and TAZ-mediated resistance to anti-cancer therapy.
Reggiani F, Gobbi G, Ciarrocchi A, Ambrosetti DC, Sancisi V. Reggiani F, et al. Biochim Biophys Acta Rev Cancer. 2020 Jan;1873(1):188341. doi: 10.1016/j.bbcan.2020.188341. Epub 2020 Jan 10. Biochim Biophys Acta Rev Cancer. 2020. PMID: 31931113 Review. - YAP/TAZ for cancer therapy: opportunities and challenges (review).
Guo L, Teng L. Guo L, et al. Int J Oncol. 2015 Apr;46(4):1444-52. doi: 10.3892/ijo.2015.2877. Epub 2015 Feb 5. Int J Oncol. 2015. PMID: 25652178 Review. - A combat with the YAP/TAZ-TEAD oncoproteins for cancer therapy.
Pobbati AV, Hong W. Pobbati AV, et al. Theranostics. 2020 Feb 18;10(8):3622-3635. doi: 10.7150/thno.40889. eCollection 2020. Theranostics. 2020. PMID: 32206112 Free PMC article. Review. - The Hippo Pathway Component TAZ Promotes Immune Evasion in Human Cancer through PD-L1.
Janse van Rensburg HJ, Azad T, Ling M, Hao Y, Snetsinger B, Khanal P, Minassian LM, Graham CH, Rauh MJ, Yang X. Janse van Rensburg HJ, et al. Cancer Res. 2018 Mar 15;78(6):1457-1470. doi: 10.1158/0008-5472.CAN-17-3139. Epub 2018 Jan 16. Cancer Res. 2018. PMID: 29339539 - Hippo pathway inhibition by blocking the YAP/TAZ-TEAD interface: a patent review.
Crawford JJ, Bronner SM, Zbieg JR. Crawford JJ, et al. Expert Opin Ther Pat. 2018 Dec;28(12):867-873. doi: 10.1080/13543776.2018.1549226. Epub 2018 Dec 2. Expert Opin Ther Pat. 2018. PMID: 30482112 Review.
Cited by
- Clinical significance of FBXW7 loss of function in human cancers.
Fan J, Bellon M, Ju M, Zhao L, Wei M, Fu L, Nicot C. Fan J, et al. Mol Cancer. 2022 Mar 26;21(1):87. doi: 10.1186/s12943-022-01548-2. Mol Cancer. 2022. PMID: 35346215 Free PMC article. Review. - Epidermal Growth Factor Receptor (EGFR) Pathway, Yes-Associated Protein (YAP) and the Regulation of Programmed Death-Ligand 1 (PD-L1) in Non-Small Cell Lung Cancer (NSCLC).
Hsu PC, Jablons DM, Yang CT, You L. Hsu PC, et al. Int J Mol Sci. 2019 Aug 5;20(15):3821. doi: 10.3390/ijms20153821. Int J Mol Sci. 2019. PMID: 31387256 Free PMC article. Review. - Research Progress of DUB Enzyme in Hepatocellular Carcinoma.
Zhao J, Guo J, Wang Y, Ma Q, Shi Y, Cheng F, Lu Q, Fu W, Ouyang G, Zhang J, Xu Q, Hu X. Zhao J, et al. Front Oncol. 2022 Jun 27;12:920287. doi: 10.3389/fonc.2022.920287. eCollection 2022. Front Oncol. 2022. PMID: 35875077 Free PMC article. Review. - CK2-induced cooperation of HHEX with the YAP-TEAD4 complex promotes colorectal tumorigenesis.
Guo Y, Zhu Z, Huang Z, Cui L, Yu W, Hong W, Zhou Z, Du P, Liu CY. Guo Y, et al. Nat Commun. 2022 Aug 25;13(1):4995. doi: 10.1038/s41467-022-32674-6. Nat Commun. 2022. PMID: 36008411 Free PMC article. - A covalent inhibitor of the YAP-TEAD transcriptional complex identified by high-throughput screening.
Nutsch K, Song L, Chen E, Hull M, Chatterjee AK, Chen JJ, Bollong MJ. Nutsch K, et al. RSC Chem Biol. 2023 Aug 29;4(11):894-905. doi: 10.1039/d3cb00044c. eCollection 2023 Nov 1. RSC Chem Biol. 2023. PMID: 37920398 Free PMC article.
References
- Society AC (2018) Cancer Facts & Figures 2018. Am. Cancer Soc DOI: 10.1182/blood-2015-12-687814 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials