Silver-Based Plasmonic Nanoparticles for and Their Use in Biosensing - PubMed (original) (raw)
Review
Silver-Based Plasmonic Nanoparticles for and Their Use in Biosensing
Alexis Loiseau et al. Biosensors (Basel). 2019.
Abstract
The localized surface plasmon resonance (LSPR) property of metallic nanoparticles is widely exploited for chemical and biological sensing. Selective biosensing of molecules using functionalized nanoparticles has become a major research interdisciplinary area between chemistry, biology and material science. Noble metals, especially gold (Au) and silver (Ag) nanoparticles, exhibit unique and tunable plasmonic properties; the control over these metal nanostructures size and shape allows manipulating their LSPR and their response to the local environment. In this review, we will focus on Ag-based nanoparticles, a metal that has probably played the most important role in the development of the latest plasmonic applications, owing to its unique properties. We will first browse the methods for AgNPs synthesis allowing for controlled size, uniformity and shape. Ag-based biosensing is often performed with coated particles; therefore, in a second part, we will explore various coating strategies (organics, polymers, and inorganics) and their influence on coated-AgNPs properties. The third part will be devoted to the combination of gold and silver for plasmonic biosensing, in particular the use of mixed Ag and AuNPs, i.e., AgAu alloys or Ag-Au core@shell nanoparticles will be outlined. In the last part, selected examples of Ag and AgAu-based plasmonic biosensors will be presented.
Keywords: LSPR; alloy; biosensors; coating; core@shell; silver nanoparticles; synthesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Figure 1
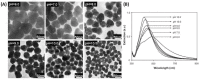
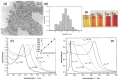
AgNPs synthesis using citrate: (A) Experimental conditions affecting the silver nanoparticle AgNPs shape and (B) TEM images of the AgNPs synthesized at different pH values: (i) 11.1, (ii) 8.3, (iii) 6.1 and (iv) 5.7. Adapted from [72]. Copyright (2009), American Chemical Society.
Figure 2
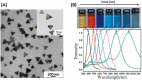
(A) TEM images and (B) UV-vis spectra of the AgNPs prepared at pH 6.0, 7.0, 8.0, 9.0, 10.0 and 10.5 by using ascorbate as reductant. Adapted from [73]. Copyright (2010), Elsevier B.V. All rights reserved.
Figure 3
TEM images of silver nanoparticles with different shapes: (A) nanospheres, (B) nanoprisms, (C) nanobars and (D) nanowires. SEM images of (E) nanocubes, (F) pyramids, (G) nanorice and (H) nanoflowers. Adapted from [54,56]. Copyright (2009), Springer Science Business Media, LLC.
Figure 4
(A) TEM image of AgNPls. (B) Dispersions of Ag (a) sphere and (b–h) nanoplate colloids with different colors and corresponding UV-vis-NIR extinction spectra that reflect the ability to tune the plasmon resonance of the nanoplates across the visible near-infrared (NIR) portion of the spectrum (500–1100 nm). The nanoplate optical resonance and size are tuned according to different rounds of Ag seed growth. Adapted from [90,93]. Copyright (2008, 2013), Royal Society of Chemistry.
Figure 5
Diagram of the nanosphere lithography (NSL) mechanism. SEM image of topography of the triangular Ag nanoparticles fabricated by NSL. Adapted from [96]. Copyright (2011), publisher and licensee Dove Medical Press Ltd.
Figure 6
(A) illustration of (I) Ag+ reduction by polyol process; (II) formation of Ag clusters; (III) seed nucleation; and (IV) seed growth into nanocubes, nanorods or nanowires, and nanospheres. SEM images of Ag nanocubes synthesized by mixing AgNO3 and polyvinyl pyrrolidone (PVP) via polyol process: (B) without and (C) sulfide-assisted synthesis (reaction time: 45 min vs. 7 min, respectively). Adapted from [98,102]. Copyright (2004), © WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. Copyright (2006), Elsevier B.V. All rights reserved.
Figure 7
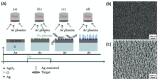
(A) Synthesis of silver nanorods by sputtering process: oxidation reduction growth (ORG). SEM images of (B) Ag nuclei and (C) AgNRs arrays. Adapted from [64].
Figure 8
Silver nanoparticles core@shell structure. Adapted from [114]. Copyright (2012), American Chemical Society.
Figure 9
(A) Illustration of natural organic matter (NOM) interactions with the surface of silver nanoparticles according to the NOM’s chemical composition and the affinity of the capping agent for the AgNP surface. Colloidal stability of (B) citrate- and (C) PVP-capped AgNPs in the absence or the presence of NOM from various origins. Adapted from [117]. Copyright (2015), American Chemical Society.
Figure 10
(A) Allylmercaptane-stabilized AgNPs: (i) core@shell morphology for allylmercaptane- (AM)-functionalized AgNPs through Ag-S chemical bonds to form the external layer, (ii) XPS spectra of Ag@AM with four different Ag/thiol ratios, and (iii) TEM images of AgNPs with Ag/AM molar ratio equal to 2/1 (AgNPs dimensions are 9 ± 3 nm and a population of NPs aggregated of 18 ± 6 nm. (B) Illustration of the encapsulation of AgNPs in thiol-modified metal-organic framework (MOF) as a host matrix. Adapted from [119,120]. Copyright (2012), American Chemical Society. Copyright (2015), Royal Society of Chemistry.
Figure 11
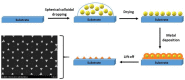
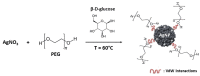
Poly(ethylene) glycol (PEG) coated method of silver nanoparticles. Inspired from [130].
Figure 12
Modified Stöber method for coating of AgNPs with silica.
Figure 13
Silica coating of Ag triangular nanoplates by (A) diaminopropane priming and (B) (3-Mercaptopropyl)triethoxysilane (MPTES) priming followed by deposition from Na2Si3O7 solution. (C) The silica shell using MPTES on triangular nanoplates (AgTNPls) allows withstanding salts without adversely affecting refractive index (RI) sensitivity in relation to original uncoated particles. Adapted from [113]. Copyright (2013), Elsevier Inc. All rights reserved.
Figure 14
(A) Effect of a hermetic SiO2 coating on the flocculation and toxicity of nanosilver particles. (B) Illustration of the nanosilver encapsulation with a hermetic SiO2 coating using hexamethyldisiloxane as silica precursor in a flame aerosol reactor. (C) TEM images of the (a,b) 1.4 wt.% and (c,d) 7.8 wt.% SiO2-coated nanosilver. Adapted from [142]. Copyright (2010), WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figure 15
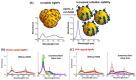
(A) Calculated extinction spectra of Ag, Au and Cu spherical NPs (20 nm) in different media. (B) Experimental extinction spectra of Ag and Au spherical NPs (10 nm) and (C) experimental response expressed as LSPR band shift of biocytin-coated Ag and Au spherical NPs (10 nm) in the presence of avidin. Adapted from [53,143]. Copyright (2014), Springer Science Business Media New York. Copyright (2012), Elsevier B.V. All rights reserved.
Figure 16
(A) Pulsed laser ablation in liquid: simultaneous ablation of Ag and Au to synthesize AgAuNPs and ablation of Au in AgNPs colloid to form core@shell structure (Au@AgNPs). Reproduced with permission from [145]. Copyright (2014,) Springer-Verlag Berlin Heidelberg. (B) Schematic illustration to form an intermediate AgAu alloy shell by interdiffusion at the NPs interface during hydrothermal treatment. Adapted from [148]. Copyright (2011), American Chemical Society.
Figure 17
(A) TEM image and (B) size histogram of spherical AgAuNPs with Au mole fraction xAu = 0.8: the average size is 18 nm. (C) Experimental and (D) calculated spectra regarding the LSPR shift of 18 nm diameter spherical AgAuNPs with varying Au molar fraction. (E) Colloidal suspensions of AuAgNPs with increasing Au concentration. Adapted from [92,144]. Copyright (1999), American Chemical Society. Copyright (2004), Elsevier B.V. All rights reserved.
Figure 18
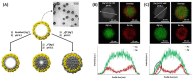
(A) Illustration of the reduction and growth process of Ag on the inner and outer surfaces of porous gold nanoshell (AuNS) with increasing amounts of Ag+ in the surrounding medium. STEM elemental mapping (Ag, Au, and overlay) of AuNS obtained after adding Ag+: (B) [Ag+] = 0.16 mM and the corresponding elemental profile along the white hatched line and (C) [Ag+] = 0.32 mM and the corresponding elemental profile along the white hatched line. The black ellipse in (C) highlights the reduction and growth of Ag at the external surface once the inner volume is completely filled. Adapted from [163]. Copyright (2019), American Chemical Society.
Figure 19
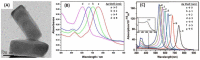
(A) TEM image of Au@Ag nanorods with 60 × 20 nm dimensions for the Au core and 4 nm thickness for the Ag shell and (B) variation of the extinction spectra of Au@Ag nanorods with varying Ag shell thickness (0–8 nm) on the 60 × 20 nm Au core. (C) The variations in the calculated LSPR spectra of Au@AgNRs with varying Ag shell thickness (0–6 nm) as well as the zoom on the spectrum allowed seeing the peak corresponding to the Au-Ag interface transversal resonance. Adapted from [154,156]. Copyright (2014), American Scientific Publishers. Copyright (2014), Springer-Verlag Wien.
Figure 20
(A) Localized surface plasmon resonance (LSPR) band shifts for Ag@Au hemispherical nanoplates (40 nm radius) supported on ITO glass with increasing RI media (a: air, b: water, c: ethanol, d: cyclohexane, e: carbon tetrachloride), and the linear relation between shift and RI (inset). (B) Evolution of the RIS with the number of Au shell electrodeposition cycles on the Ag core for the previous Ag@Au nanoplates. (C) The evolution of the RIS with the concentration of AgNO3 for the deposition of the Ag shell on the 20 × 60 nm Au core in the case of Au@AgNRs (TEM image was previously shown in Figure 19A). Adapted from [152,156]. Copyright (2013), American Chemical Society. Copyright (2014), Springer-Verlag Wien.
Figure 21
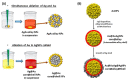
(A) The structural evolution of AuAg nanostructures during the galvanic replacement reaction upon addition of HAuCl4 and (B) absorption spectra evolution as a function of time of AgNPs titrated with increasing volumes of HAuCl4 to form AuNS: the LSPR band gradually shifts through the whole visible spectrum toward NIR wavelengths. Adapted from [175]. Copyright (2018), American Chemical Society.
Figure 22
Silver triangular nanoparticles fabricated by NSL on a glass substrate. (A) Tapping mode AFM image of the Ag triangular NPs. (B) Surface chemistry of the Ag nanobiosensor. A mixed monolayer of (1) 11-MUA and (2) 1-OT is formed on the exposed surfaces of the AgNPs followed by the covalent linking of (3) biotin to the carboxyl groups of (1) 11-MUA. Schematic illustration of (C) streptavidin binding to a biotinylated Ag nanobiosensor and (D) biotin covalently linked to the Ag nanobiosensor surface while antibiotin-labeled AuNPs are subsequently exposed to the surface. LSPR spectra (E) before (solid black) and after (dashed blue) binding of native antibiotin and (F) before (solid black) and after (dashed red) binding of antibiotin-labeled NPs. Adapted from [57,177]. Copyright (2002, 2011), American Chemical Society.
Figure 23
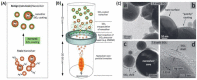
(A) Schematic illustration of the experimental set-up used for the LSPR optical fiber sensor. (B) SEM image of immobilized AgNPs on optical fiber surface. (C) Illustration of the employed strategy for the development of LSPR optical fiber biosensors based on AgNPs. Adapted from [179].
Figure 24
(A,B) Schematic illustration showing the preparation of glass-supported core@shell NPs for SA biosensing. (C) SEM image of Ag@Au hemispherical nanoplates supported on ITO glass. (D) LSPR peak (500 nm) was shifted upon successive treatments with APTMS, biotin and SA. (E) Relationship between the LSPR band shift and SA concentration for Ag@Au NPls. (F) SEM image of Au@AgTNPls supported on ITO glass. (G) LSPR peak (700 nm) shifted upon successive treatment with APTMS, biotin and SA. (H) Linear relationship between the LSPR band shift and SA concentration. Adapted from [152,157]. Copyright (2013), American Chemical Society. Copyright (2013), Springer Science Business Media New York.
Figure 25
(A) Principle of nanoparticle aggregation based-colorimetric assay for the melamine detection with dopamine-modified AgNPs. (B) UV-Vis spectra of dopamine-stabilized AgNPs suspensions with different melamine concentrations: (1) 0 mM, (2) 0.08 mM, (3) 0.4 mM, (4) 2 mM, (5) 8 mM and (6) 10 mM. Adapted from [184]. Copyright (2011), Royal Society of Chemistry.
Figure 26
DNA detection by naked-eye readout with the silver reduction on gold nanostars [188]. Copyright (2015), Elsevier B.V. All rights reserved.
Figure 27
Schematic illustration of the preparation of the (A) reporter probe and (B) capture probe as well as the principle of the colorimetric detection of illicit drug based on non-aggregation Au@Ag core@shell NPs. Reproduced with permission from [189]. Copyright (2017), Published by Elsevier B.V.
Figure 28
(A) Illustration of a metal-enhanced sandwich immunoassay on silver island films (SIFs). Fluorescence emission of the Rhodamine Red-X-labeled anti-myoglobin antibody attached to the surface-immobilized myoglobin (B) for a given myoglobin concentration (100 ng/mL) on SIFs and on glass, and (C) at different myoglobin concentrations on SIFs. Adapted from [191]. Copyright (2005), Elsevier Ltd. All rights reserved.
Figure 29
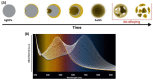
(A) Illustration of the strategy to modify the AgNPs shape from nanoprisms to nanodiscs through Ag oxidation for colorimetric sensing of glucose. SEM images of the Ag nanoprisms before and after incubation with glucose oxidase and glucose (100 μM) for 60 min are also showed. (B) Absorption spectra of the Ag nanoprisms after glucose incubation in various concentrations for 40 min with photographs of the corresponding suspensions. Adapted from [196]. Copyright (2013), American Chemical Society.
Figure 30
(A) Illustration showing the oxidation of Ag shell on Au@Ag core@shell NPs to Ag+ in the presence of H2O2 produced by an enzymatic reaction. (B) Schematic illustration showing the glucose sensing mechanism with Ag/Au nanoshells. Adapted from [198]. Copyright (2012) WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Similar articles
- Gold and silver nanoparticles in sensing and imaging: sensitivity of plasmon response to size, shape, and metal composition.
Lee KS, El-Sayed MA. Lee KS, et al. J Phys Chem B. 2006 Oct 5;110(39):19220-5. doi: 10.1021/jp062536y. J Phys Chem B. 2006. PMID: 17004772 - Core-Shell Gold/Silver Nanoparticles for Localized Surface Plasmon Resonance-Based Naked-Eye Toxin Biosensing.
Loiseau A, Zhang L, Hu D, Salmain M, Mazouzi Y, Flack R, Liedberg B, Boujday S. Loiseau A, et al. ACS Appl Mater Interfaces. 2019 Dec 18;11(50):46462-46471. doi: 10.1021/acsami.9b14980. Epub 2019 Dec 5. ACS Appl Mater Interfaces. 2019. PMID: 31744295 - Plasmonic properties of silver nanostructures coated with an amorphous silicon-carbon alloy and their applications for sensitive sensing of DNA hybridization.
Touahir L, Galopin E, Boukherroub R, Gouget-Laemmel AC, Chazalviel JN, Ozanam F, Saison O, Akjouj A, Pennec Y, Djafari-Rouhani B, Szunerits S. Touahir L, et al. Analyst. 2011 May 7;136(9):1859-66. doi: 10.1039/c0an01022g. Epub 2011 Mar 24. Analyst. 2011. PMID: 21437320 - Self-assembled plasmonic nanostructures.
Klinkova A, Choueiri RM, Kumacheva E. Klinkova A, et al. Chem Soc Rev. 2014 Jun 7;43(11):3976-91. doi: 10.1039/c3cs60341e. Epub 2014 Mar 6. Chem Soc Rev. 2014. PMID: 24599020 Review. - Stabilization of Silver and Gold Nanoparticles: Preservation and Improvement of Plasmonic Functionalities.
Kang H, Buchman JT, Rodriguez RS, Ring HL, He J, Bantz KC, Haynes CL. Kang H, et al. Chem Rev. 2019 Jan 9;119(1):664-699. doi: 10.1021/acs.chemrev.8b00341. Epub 2018 Oct 22. Chem Rev. 2019. PMID: 30346757 Review.
Cited by
- Hemolytic Activity of Nanoparticles as a Marker of Their Hemocompatibility.
Yedgar S, Barshtein G, Gural A. Yedgar S, et al. Micromachines (Basel). 2022 Nov 27;13(12):2091. doi: 10.3390/mi13122091. Micromachines (Basel). 2022. PMID: 36557391 Free PMC article. Review. - Emerging Applications of Nanobiosensors in Pathogen Detection in Water and Food.
Valenzuela-Amaro HM, Aguayo-Acosta A, Meléndez-Sánchez ER, de la Rosa O, Vázquez-Ortega PG, Oyervides-Muñoz MA, Sosa-Hernández JE, Parra-Saldívar R. Valenzuela-Amaro HM, et al. Biosensors (Basel). 2023 Oct 11;13(10):922. doi: 10.3390/bios13100922. Biosensors (Basel). 2023. PMID: 37887115 Free PMC article. Review. - Green Synthesis of Silver Nanoparticles with Extracts from Kalanchoe fedtschenkoi: Characterization and Bioactivities.
Mejía-Méndez JL, Sánchez-Ante G, Cerro-López M, Minutti-Calva Y, Navarro-López DE, Lozada-Ramírez JD, Bach H, López-Mena ER, Sánchez-Arreola E. Mejía-Méndez JL, et al. Biomolecules. 2024 Jun 30;14(7):782. doi: 10.3390/biom14070782. Biomolecules. 2024. PMID: 39062496 Free PMC article. - Transcriptome Profile Alterations with Carbon Nanotubes, Quantum Dots, and Silver Nanoparticles: A Review.
Horstmann C, Davenport V, Zhang M, Peters A, Kim K. Horstmann C, et al. Genes (Basel). 2021 May 23;12(6):794. doi: 10.3390/genes12060794. Genes (Basel). 2021. PMID: 34070957 Free PMC article. Review. - Noble Metals and Soft Bio-Inspired Nanoparticles in Retinal Diseases Treatment: A Perspective.
De Matteis V, Rizzello L. De Matteis V, et al. Cells. 2020 Mar 10;9(3):679. doi: 10.3390/cells9030679. Cells. 2020. PMID: 32164376 Free PMC article. Review.
References
- Khaydarov R.R., Khaydarov R.A., Estrin Y., Evgrafova S., Scheper T., Endres C., Cho S.Y. Nanomaterials: Risks and Benefits. Springer; Dordrecht, The Netherlands: 2009. Silver nanoparticles; pp. 287–297.
- Marambio-Jones C., Hoek E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010;12:1531–1551. doi: 10.1007/s11051-010-9900-y. - DOI
- Quang Huy T., van Quy N., Anh-Tuan L. Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013;4:033001. doi: 10.1088/2043-6262/4/3/033001. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources