A randomised controlled trial of an Intervention to Improve Compliance with the ARRIVE guidelines (IICARus) - PubMed (original) (raw)
A randomised controlled trial of an Intervention to Improve Compliance with the ARRIVE guidelines (IICARus)
Kaitlyn Hair et al. Res Integr Peer Rev. 2019.
Abstract
Background: The ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines are widely endorsed but compliance is limited. We sought to determine whether journal-requested completion of an ARRIVE checklist improves full compliance with the guidelines.
Methods: In a randomised controlled trial, manuscripts reporting in vivo animal research submitted to PLOS ONE (March-June 2015) were randomly allocated to either requested completion of an ARRIVE checklist or current standard practice. Authors, academic editors, and peer reviewers were blinded to group allocation. Trained reviewers performed outcome adjudication in duplicate by assessing manuscripts against an operationalised version of the ARRIVE guidelines that consists 108 items. Our primary outcome was the between-group differences in the proportion of manuscripts meeting all ARRIVE guideline checklist subitems.
Results: We randomised 1689 manuscripts (control: n = 844, intervention: n = 845), of which 1269 were sent for peer review and 762 (control: n = 340; intervention: n = 332) accepted for publication. No manuscript in either group achieved full compliance with the ARRIVE checklist. Details of animal husbandry (ARRIVE subitem 9b) was the only subitem to show improvements in reporting, with the proportion of compliant manuscripts rising from 52.1 to 74.1% (X 2 = 34.0, df = 1, p = 2.1 × 10-7) in the control and intervention groups, respectively.
Conclusions: These results suggest that altering the editorial process to include requests for a completed ARRIVE checklist is not enough to improve compliance with the ARRIVE guidelines. Other approaches, such as more stringent editorial policies or a targeted approach on key quality items, may promote improvements in reporting.
Keywords: ARRIVE; Randomised controlled trial; Reporting guidelines.
Conflict of interest statement
Competing interestsES and MM are in receipt of competitive research grants from the NC3Rs who developed the ARRIVE guidelines. SL was funded by an NC3Rs PhD studentship. ES, MM, DH, and NK are members of an NC3Rs working group to review the ARRIVE guidelines (https://www.nc3rs.org.uk/revision-arrive-guidelines). CM, GM, AC, GA, and MD were all editors at PLOS ONE throughout the duration of the study. ES is Editor-in-Chief at BMJ Open Science. All other authors have no other competing interests to declare.
Figures
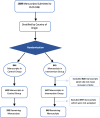
Fig. 1
Manuscript processing
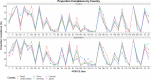
Fig. 2
Percentage compliance for each ARRIVE subitem; percentage compliance for each ARRIVE subitem with 95% confidence intervals; asterisk denotes statistical significance; figure divided into article sections specified in the ARRIVE guidelines
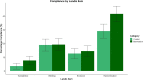
Fig. 3
Compliance by country; percentage compliance for each ARRIVE subitem for manuscripts in each country (for countries with N manuscripts ≥10)
Fig. 4
Landis 4 individual compliance; percentage compliance for each Landis criteria present in the ARRIVE guidelines with 95% confidence intervals
Similar articles
- Effect of an editorial intervention to improve the completeness of reporting of randomised trials: a randomised controlled trial.
Blanco D, Schroter S, Aldcroft A, Moher D, Boutron I, Kirkham JJ, Cobo E. Blanco D, et al. BMJ Open. 2020 May 18;10(5):e036799. doi: 10.1136/bmjopen-2020-036799. BMJ Open. 2020. PMID: 32430454 Free PMC article. Clinical Trial. - The ARRIVE guidelines 2.0: updated guidelines for reporting animal research.
Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, Petersen OH, Rawle F, Reynolds P, Rooney K, Sena ES, Silberberg SD, Steckler T, Würbel H. Percie du Sert N, et al. J Physiol. 2020 Sep;598(18):3793-3801. doi: 10.1113/JP280389. Epub 2020 Jul 14. J Physiol. 2020. PMID: 32666574 Free PMC article. - The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research.
Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl U, Emerson M, Garner P, Holgate ST, Howells DW, Karp NA, Lazic SE, Lidster K, MacCallum CJ, Macleod M, Pearl EJ, Petersen OH, Rawle F, Reynolds P, Rooney K, Sena ES, Silberberg SD, Steckler T, Würbel H. Percie du Sert N, et al. PLoS Biol. 2020 Jul 14;18(7):e3000410. doi: 10.1371/journal.pbio.3000410. eCollection 2020 Jul. PLoS Biol. 2020. PMID: 32663219 Free PMC article. - Reporting in rodent models of 'chemobrain': a systematic review assessing compliance with the ARRIVE guidelines.
George RP, Semendric I, Bowley-Schubert ER, Chivonivoni CT, Warrender AP, Whittaker AL. George RP, et al. Support Care Cancer. 2021 Nov;29(11):7073-7084. doi: 10.1007/s00520-021-06312-8. Epub 2021 Jun 2. Support Care Cancer. 2021. PMID: 34080055 Review. - Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals.
Turner L, Shamseer L, Altman DG, Weeks L, Peters J, Kober T, Dias S, Schulz KF, Plint AC, Moher D. Turner L, et al. Cochrane Database Syst Rev. 2012 Nov 14;11(11):MR000030. doi: 10.1002/14651858.MR000030.pub2. Cochrane Database Syst Rev. 2012. PMID: 23152285 Free PMC article. Review.
Cited by
- The ARRIVE 2.0 Guidelines: Importance and Full Adoption by AALAS Journals.
Suckow MA, Fallon MT. Suckow MA, et al. J Am Assoc Lab Anim Sci. 2024 Sep 5;63(5):449-54. doi: 10.30802/AALAS-JAALAS-24-083. Online ahead of print. J Am Assoc Lab Anim Sci. 2024. PMID: 39237288 No abstract available. - Enhancing reporting through structure: a before and after study on the effectiveness of SPIRIT-based templates to improve the completeness of reporting of randomized controlled trial protocols.
Blanco D, Donadio MVF, Cadellans-Arróniz A. Blanco D, et al. Res Integr Peer Rev. 2024 May 31;9(1):6. doi: 10.1186/s41073-024-00147-7. Res Integr Peer Rev. 2024. PMID: 38816752 Free PMC article. - A minimal metadata set (MNMS) to repurpose nonclinical in vivo data for biomedical research.
Moresis A, Restivo L, Bromilow S, Flik G, Rosati G, Scorrano F, Tsoory M, O'Connor EC, Gaburro S, Bannach-Brown A. Moresis A, et al. Lab Anim (NY). 2024 Mar;53(3):67-79. doi: 10.1038/s41684-024-01335-0. Epub 2024 Mar 4. Lab Anim (NY). 2024. PMID: 38438748 Free PMC article. Review. - Poor statistical reporting, inadequate data presentation and spin persist despite Journal awareness and updated Information for Authors.
Héroux M, Diong J, Bye E, Fisher G, Robertson L, Butler A, Gandevia S. Héroux M, et al. F1000Res. 2023 Nov 20;12:1483. doi: 10.12688/f1000research.142841.1. eCollection 2023. F1000Res. 2023. PMID: 38434651 Free PMC article. - Letter to the Editor: Editorial: In Musculoskeletal Research, Too Many Animals are Being Harmed for Too Small a Return.
Smith A. Smith A. Clin Orthop Relat Res. 2024 May 1;482(5):896-898. doi: 10.1097/CORR.0000000000003013. Epub 2024 Feb 16. Clin Orthop Relat Res. 2024. PMID: 38363557 No abstract available.
References
- Avey MT, Moher D, Sullivan KJ, Fergusson D, Griffin G, Grimshaw JM, Hutton B, Lalu MM, Macleod M, Marshall J, Mei SHJ, Rudnicki M, Stewart DJ, Turgeon AF, Mcintyre L, Canadian Critical Care Translational Biology, G The devil is in the details: incomplete reporting in preclinical animal research. PLoS One. 2016;11:e0166733. doi: 10.1371/journal.pone.0166733. - DOI - PMC - PubMed
- Cobo E, Cortes J, Ribera JM, Cardellach F, Selva-O’Callaghan A, Kostov B, Garcia L, Cirugeda L, Altman DG, Gonzalez JA, Sanchez JA, Miras F, Urrutia A, Fonollosa V, Rey-Joly C, Vilardell M. Effect of using reporting guidelines during peer review on quality of final manuscripts submitted to a biomedical journal: masked randomised trial. BMJ. 2011;343:d6783. doi: 10.1136/bmj.d6783. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous