Microbiota maintain colonic homeostasis by activating TLR2/MyD88/PI3K signaling in IL-10-producing regulatory B cells - PubMed (original) (raw)
. 2019 Jun 18;129(9):3702-3716.
doi: 10.1172/JCI93820.
Akihiko Oka 1 2, Bo Liu 1, Jeremy W Herzog 1, Chang Soo Eun 1 3, Ting-Jia Fan 1 4, Emily Bulik-Sullivan 5, Ian M Carroll 1 5, Jonathan J Hansen 1 4, Liang Chen 6, Justin E Wilson 6, Nancy C Fisher 4, Jenny Py Ting 6, Tomonori Nochi 7 8, Angela Wahl 7, J Victor Garcia 7, Christopher L Karp 9, R Balfour Sartor 1 4
Affiliations
- PMID: 31211700
- PMCID: PMC6715367
- DOI: 10.1172/JCI93820
Microbiota maintain colonic homeostasis by activating TLR2/MyD88/PI3K signaling in IL-10-producing regulatory B cells
Yoshiyuki Mishima et al. J Clin Invest. 2019.
Abstract
Resident microbiota activate regulatory cells that modulate intestinal inflammation and promote and maintain intestinal homeostasis. IL-10 is a key mediator of immune regulatory function. Our studies described the functional importance and mechanisms by which gut microbiota and specific microbial components influenced the development of intestinal IL-10-producing B cells. We used fecal transplant to germ-free (GF) Il10+/EGFP reporter and Il10-/- mice to demonstrate that microbiota from specific pathogen-free mice primarily stimulated IL-10-producing colon-specific B cells and T regulatory-1 cells in ex-GF mice. IL-10 in turn down-regulated microbiota-activated mucosal inflammatory cytokines. TLR2/9 ligands and enteric bacterial lysates preferentially induced IL-10 production and regulatory capacity of intestinal B cells. Analysis of Il10+/EGFP mice crossed with additional gene-deficient strains and B cell co-transfer studies demonstrated that microbiota-induced IL-10-producing intestinal B cells ameliorated chronic T cell-mediated colitis in a TLR2, MyD88 and PI3K-dependent fashion. In vitro studies implicated PI3Kp110δ and AKT downstream signaling. These studies demonstrated that resident enteric bacteria activated intestinal IL-10-producing B cells through TLR2, MyD88 and PI3K pathways. These B cells reduced colonic T cell activation and maintained mucosal homeostasis in response to intestinal microbiota.
Keywords: B cells; Gastroenterology; Immunology; Inflammatory bowel disease; T cells.
Conflict of interest statement
Conflict of interest: The authors have declared that no conflict of interest exists.
Figures
Figure 1. Resident intestinal microbiota increases the frequency of intestinal IL-10–producing immune cells and enhance IL-10 production.
(A) Left: Spontaneous IL-10 secretion by colonic tissue explants; middle: number of total IL-10–producing (GFP+) colon LP cells; right: Il10 mRNA expression in distal colon tissue normalized by expression in GF mice in GF, SPF-raised, or GF-conventionalized Il10+/EGFP reporter mice 3 days and 7 days (D3 and D7) after fecal transplantation (TP) with SPF feces. n = 6–9 mice/group, combined from 2 independent experiments. (B and C) Phosphorylation levels of STAT3 in the distal colon were evaluated by Western blot analysis and quantified by densitometry. n = 4 mice/group. (D) IL-10–producing (GFP+) colon LP cells were characterized by flow cytometry identifying cell subsets with antibodies to the indicated cell surface proteins. CD25–CD4+ T cells (CD25–CD4+CD3+), B cells (B220+CD19+), and Tregs, including GFP+Foxp3+RORγt+CD4+ T cells within the total GFP+Foxp3+CD4+ T cell population. n = 5–7 mice/group, combined from 2 independent experiments. (E) Representative dot plots for colon LP GFP+CD25–CD4+ T cells (CD4+ T cell–gated) and GFP+ B cells (B cell–gated) in GF and conventionalized day 7 Il10+/EGFP mice. For flow cytometry, the GFP+ population in live CD45+ colon LP cells was assessed using WT (GFP–) cells stained with the same antibodies as target samples as a control (see Flow cytometry in Methods). All data are presented as median values; *P < 0.05, **P < 0.01, ***P < 0.001, Kruskal-Wallis test with Dunn’s post hoc test.
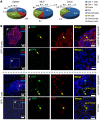
Figure 2. Distribution and characteristics of SPF mucosal and splenic GFP+ T and B cells.
Physiological IL-10–producing immune cells were characterized in colonic LP, MLN, and spleen from SPF-raised Il10+/EGFP reporter mice. (A) Percentages of cell types among total GFP+ cells were assessed by flow cytometry using antibodies to cell surface markers as described in Methods, Flow cytometry. (B) Immunofluorescence was performed on distal colon tissue from SPF-reared Il10+/EGFP mice to characterize the distribution of GFP+ B cells and GFP+ T cells in lymphoid aggregates and the LP of the distal colon. GFP+ cells are shown in green. B cells (B220+) and T cells (CD3+) are shown in red. Merged images show B and T cells that are GFP+. Scale bars: far left column, 50 mm; right panels, 20 mm.
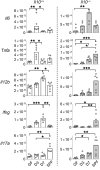
Figure 3. Bacterial colonization influences the gene expression kinetics of the majority of proinflammatory cytokines in an IL-10–dependent fashion.
Kinetic expression of selected genes in distal colons from GF, SPF-raised (SPF), and ex-GF conventionalized (3 and 7 days after fecal transplantation) Il10+/EGFP reporter mice (Il10+/+) or _Il10–/–_mice was determined by real-time PCR of colonic tissues. All gene expression was normalized with Actb. n = 6 mice/group, combined from 2 independent experiments. Data are presented as median; *P < 0.05, **P < 0.01, ***P < 0.001, Kruskal-Wallis test with Dunn’s post hoc test.
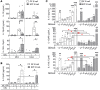
Figure 4. CBL stimulates IL-10–producing colonic LP B cells ex vivo.
Unfractionated colonic LP cells from GF or SPF-reared Il10+/EGFP reporter mice were cultured with 0–100 μg/mL CBL from SPF mice for 2 days, as indicated. (A) The frequency of GFP+ cells was assessed by flow cytometry. conc., concentration. (B) Representative dot plots for GFP+ cells in GF colonic cells stimulated with 0 (CBL–), 10, 50, or 100 μg/mL CBL. (C) Using flow cytometry, we determined frequencies of GFP-expressing CD4+ T cells (CD4+CD3+) or B cells (CD19+B220+) as a percentage of total T or B cells using control WT (GFP–) cells that stained with the same antibodies as target samples. (D) Supernatant levels of IL-10 and IL-12p40 were measured by ELISA, and IL-10/IL12 p40 ratios were calculated. Data are presented as median of 8 separated cell cultures, with cells in each culture pooled from 2–4 mice, combined from 3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, Kruskal-Wallis test with Dunn’s post hoc test.
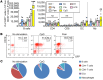
Figure 5. Bacterial products predominantly expand GFP+ B cells and innate immune cells ex vivo.
Unfractionated colonic LP cells from GF Il10+/EGFP reporter mice were cultured without (–, media only) or with 200 ng/mL LPS, 50 ng/mL Pam3csk (Pam), 1 nM CpG-DNA (CpG), 10 μg/mL lysates of E. coli LF82 (Ec), E. faecalis (Ef), or R. gnavus (Rg) or a mixture of 17 strains of Clostridia species (Clo). (A) Frequencies of GFP-expressing cell types were determined by flow cytometry using antibodies to cell surface markers as described in Methods. Data are presented as median of 4 separate cell cultures, with cells in each culture pooled from 2–4 mice; *P < 0.05, **P < 0.01, ***P < 0.001 (vs. no stimulation), Kruskal-Wallis test with Dunn’s post hoc test. (B) Representative dot plots for GFP (IL-10)+ B cells stimulated with or without CpG or Pam3. (C) Summary pie charts for cell populations expressing GFP.
Figure 6. Enteric resident bacteria in mice increase the capacity of intestinal B cells to suppress ex vivo bacterial lysate–stimulated inflammatory cytokine production that is mediated by IL-10 signaling.
(A) Cytokine secretion by 10 μg/mL CBL-stimulated colonic LP B cells isolated from GF or SPF-raised WT mice in culture for 2 days. Cytokine concentrations were measured by ELISA. Data are presented as median of 6–7 separate cell cultures, with cells in each culture pooled from 3–4 mice. Mann-Whitney U test (GF vs. SPF). *P < 0.05, **P < 0.01, ***P < 0.001. (B) Colonic B cells from GF or SPF-reared WT mice were cultured with anti–IL-10R or isotype control antibodies in the presence or absence of 10 μg/mL CBL for 24 hours. Supernatant levels of IL-12p40 were measured by ELISA. Data are presented as median of 4 separate cell cultures, with cells in each culture pooled from 2–4 mice; *P < 0.05, Kruskal-Wallis test with Dunn’s post hoc test. (C) Cytokine secretion by MLN B cells from GF or SPF-raised WT mice cultured for 2 days with the indicated bacteria lysates or TLR ligands (see Figure 5 legend). IL-10 and IL-12p40 in culture supernatants were measured by ELISA. Data are presented as median of 4 separate cell cultures, with cells in each culture pooled from 3–6 mice. *P < 0.05, **P < 0.01, ***P < 0.001 (vs. no stimulation), Kruskal-Wallis test with Dunn’s post hoc test; #P < 0.05, ##P < 0.01 (GF vs. SPF), Mann-Whitney U test.
Figure 7. TLR2/MyD88 signaling increases the frequency of IL-10–producing B cells upon bacterial product stimulation ex vivo and mediates the suppression of experimental colitis by IL-10–producing B cells in vivo.
(A) Colonic LP B cells from 8- to 10-week-old WT Il10+/EGFP, _Tlr2_−/− Il10+/EGFP, _Tlr4_−/− Il10+/EGFP, and _Myd88_−/− Il10+/EGFP mice were cultured without (–) or with 10 μg/mL CBL, 50 ng/mL Pam3, or 200 ng/mL LPS for 24 hours, after which IL-10 levels in culture supernatants were measured by ELISA (top panel). GFP+ populations in B cells (live CD45+CD19+B220+) were analyzed with flow cytometry in reference to WT (GFP–) control cells stained with the same antibodies as target samples. Data are presented as median of 7–8 separate cell cultures, with cells in each culture pooled from 2–3 mice, combined from 3 independent experiments (bottom panel). *P < 0.05, **P < 0.01. (B) Eight-week-old GF Il10+/EGFP reporter mice were conventionalized with SPF fecal bacterial transplantation. Mice were given anti-TLR2 antibody (αTLR2) or isotype control i.v. on days 0 and 4 after fecal bacterial transplantation and harvested on day 7 for IL-10 analysis. Colonic LP cells were isolated, and live CD45+GFP+ B cells (CD19+B220+) were analyzed by flow cytometry (left) as described above; Il10 mRNA levels were normalized with Actb (right). n = 4–5 mice. Data are presented as median, Mann-Whitney U test. (C and D) Splenic B cells (1 × 106) from SPF-raised WT, _Tlr2_−/−, _Tlr4_−/−, _Myd88_−/−, or _Il10_−/− mice were cotransferred with 5 × 105 WT naive CD4+ T cells isolated by a naive CD4+ T cell isolation kit (see Methods, Cell purification) into SPF _Rag2_−/− _Il10_−/− recipients. Six weeks after cell transfer, mice were evaluated for (C) severity of colitis by histology and (D) measurement of spontaneously secreted IL-10 and IFN-γ in colonic tissue explants by ELISA. n = 7–9 mice/group, combined from 2 independent experiments. Data are presented as median; *P < 0.05 and **P < 0.01 (vs. WT mice), Kruskal-Wallis test with Dunn’s post hoc test.
Figure 8. The PI3K/AKT pathway is involved in TLR2-dependent IL-10 production by CBL-stimulated B cells and their regulatory effect on T cell–mediated colitis.
Splenic B cells (1 × 106/well) from 8- to 10-week-old SPF WT, _Tlr2_−/−, and _Tlr4_−/− mice were cultured without or with 10 μg/mL CBL. Cells were harvested 0, 15, 30, and 60 minutes after CBL stimulation, and phosphorylation of AKT was analyzed by Western blot. (A) Representative blots and (B) densitometric analysis of phospho-AKT/AKT are shown. n = 5 mice/group, combined from 2 independent experiments. *P < 0.05, **P < 0.01, Kruskal-Wallis test with Dunn’s post hoc test. (C) Splenic B cells (1 × 106/well) from 8- to 10-week-old SPF Il10+/EGFP mice were cultured with 1 μM pan-PI3K inhibitor wortmannin (Wort) or Ly294002 (Ly), 1 μM PI3Kp110δ-specific inhibitor IC-87114 (IC), or DMSO alone, with stimulation with 10 μg/mL CBL for 48 hours. Supernatant levels of IL-10 and IL-12p40 were measured by ELISA. (D) GFP+ population in B cells (live CD45+CD19+B220+) were analyzed by flow cytometry in reference to WT (GFP–) control cells stained with the same antibodies as target samples. Data are presented as median of 6 separate cell cultures, combined from 2 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, Kruskal-Wallis test with Dunn’s post hoc test. (E) Splenic B cells (5 × 105/well) from 8- to 10-week-old SPF WT and PI3Kp110δKI mice were cultured with 10 μg/mL CBL for 48 hours. Supernatant levels of IL-10 were measured by ELISA. Data are presented as median. n = 4 mice, combined from 2 independent experiments. *P < 0.05, Mann-Whitney U test. (F) Splenic B cells (1 × 106) from SPF-raised WT or PI3Kp110δKI mice were cotransferred with WT naive CD4+ T cells (5 × 105) isolated by a naive CD4+ T cell isolation kit into SPF _Rag2_−/− _Il10_−/− recipients. Six weeks after cell transfer, mice were evaluated for severity of colitis by histology and measurement of spontaneously secreted IL-10 levels in colonic tissue explants by ELISA. n =7–9 mice/ group, combined from 2 independent experiments. Data are presented as median; *P < 0.05, Mann-Whitney U test.
Similar articles
- Phosphoinositide 3-Kinase P110δ-Signaling Is Critical for Microbiota-Activated IL-10 Production by B Cells that Regulate Intestinal Inflammation.
Oka A, Mishima Y, Liu B, Herzog JW, Steinbach EC, Kobayashi T, Plevy SE, Sartor RB. Oka A, et al. Cells. 2019 Sep 21;8(10):1121. doi: 10.3390/cells8101121. Cells. 2019. PMID: 31546615 Free PMC article. - Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon.
Jeon SG, Kayama H, Ueda Y, Takahashi T, Asahara T, Tsuji H, Tsuji NM, Kiyono H, Ma JS, Kusu T, Okumura R, Hara H, Yoshida H, Yamamoto M, Nomoto K, Takeda K. Jeon SG, et al. PLoS Pathog. 2012;8(5):e1002714. doi: 10.1371/journal.ppat.1002714. Epub 2012 May 31. PLoS Pathog. 2012. PMID: 22693446 Free PMC article. - Induction of bacterial antigen-specific colitis by a simplified human microbiota consortium in gnotobiotic interleukin-10-/- mice.
Eun CS, Mishima Y, Wohlgemuth S, Liu B, Bower M, Carroll IM, Sartor RB. Eun CS, et al. Infect Immun. 2014 Jun;82(6):2239-46. doi: 10.1128/IAI.01513-13. Epub 2014 Mar 18. Infect Immun. 2014. PMID: 24643531 Free PMC article. - Regulatory immune cells in regulation of intestinal inflammatory response to microbiota.
Sun M, He C, Cong Y, Liu Z. Sun M, et al. Mucosal Immunol. 2015 Sep;8(5):969-978. doi: 10.1038/mi.2015.49. Epub 2015 Jun 17. Mucosal Immunol. 2015. PMID: 26080708 Free PMC article. Review. - Strategies to Dissect Host-Microbial Immune Interactions That Determine Mucosal Homeostasis vs. Intestinal Inflammation in Gnotobiotic Mice.
Rogala AR, Oka A, Sartor RB. Rogala AR, et al. Front Immunol. 2020 Feb 18;11:214. doi: 10.3389/fimmu.2020.00214. eCollection 2020. Front Immunol. 2020. PMID: 32133003 Free PMC article. Review.
Cited by
- The Emulsifier Carboxymethylcellulose Induces More Aggressive Colitis in Humanized Mice with Inflammatory Bowel Disease Microbiota Than Polysorbate-80.
Rousta E, Oka A, Liu B, Herzog J, Bhatt AP, Wang J, Habibi Najafi MB, Sartor RB. Rousta E, et al. Nutrients. 2021 Oct 12;13(10):3565. doi: 10.3390/nu13103565. Nutrients. 2021. PMID: 34684567 Free PMC article. - Convergent inducers and effectors of T cell paralysis in the tumour microenvironment.
Hanahan D, Michielin O, Pittet MJ. Hanahan D, et al. Nat Rev Cancer. 2025 Jan;25(1):41-58. doi: 10.1038/s41568-024-00761-z. Epub 2024 Oct 24. Nat Rev Cancer. 2025. PMID: 39448877 Review. - Claudin-2 upregulation enhances intestinal permeability, immune activation, dysbiosis, and mortality in sepsis.
Oami T, Abtahi S, Shimazui T, Chen CW, Sweat YY, Liang Z, Burd EM, Farris AB, Roland JT, Tsukita S, Ford ML, Turner JR, Coopersmith CM. Oami T, et al. Proc Natl Acad Sci U S A. 2024 Mar 5;121(10):e2217877121. doi: 10.1073/pnas.2217877121. Epub 2024 Feb 27. Proc Natl Acad Sci U S A. 2024. PMID: 38412124 Free PMC article. - Microbial-Based and Microbial-Targeted Therapies for Inflammatory Bowel Diseases.
Oka A, Sartor RB. Oka A, et al. Dig Dis Sci. 2020 Mar;65(3):757-788. doi: 10.1007/s10620-020-06090-z. Dig Dis Sci. 2020. PMID: 32006212 Free PMC article. Review. - Intestinal Flora and Disease Mutually Shape the Regional Immune System in the Intestinal Tract.
Zhou B, Yuan Y, Zhang S, Guo C, Li X, Li G, Xiong W, Zeng Z. Zhou B, et al. Front Immunol. 2020 Apr 3;11:575. doi: 10.3389/fimmu.2020.00575. eCollection 2020. Front Immunol. 2020. PMID: 32318067 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA156330/CA/NCI NIH HHS/United States
- P40 OD010995/OD/NIH HHS/United States
- R01 AI123010/AI/NIAID NIH HHS/United States
- P30 DK034987/DK/NIDDK NIH HHS/United States
- P30 CA016086/CA/NCI NIH HHS/United States
- R01 AI140799/AI/NIAID NIH HHS/United States
- R01 DK053347/DK/NIDDK NIH HHS/United States
- R01 AI111899/AI/NIAID NIH HHS/United States
- P30 DK056350/DK/NIDDK NIH HHS/United States
- R01 MH108179/MH/NIMH NIH HHS/United States
- P01 DK094779/DK/NIDDK NIH HHS/United States