The Influence of Chemical Structure and the Presence of Ascorbic Acid on Anthocyanins Stability and Spectral Properties in Purified Model Systems - PubMed (original) (raw)
The Influence of Chemical Structure and the Presence of Ascorbic Acid on Anthocyanins Stability and Spectral Properties in Purified Model Systems
Rachel Levy et al. Foods. 2019.
Abstract
The loss of color pigment is an important quality factor of food products. This work aimed to systematically study, in purified model systems, the influence of anthocyanins' structure (by increasing the size of the conjugated sugar) and the presence of ascorbic acid on their stability and spectral properties during storage at two pH levels relevant to medium and high acid foods (6.5 and 4.5, respectively). Anthocyanins (cyanidin (Cy), cyanidin 3-O-β-glucoside (Cy3G) and cyanidin 3-O-β-rutinoside (Cy3R)) displayed first-order degradation rates, presenting higher stability in acidic medium and enhanced stability with increasing size of conjugated sugar. The addition of ascorbic acid resulted in significantly enhanced degradation. Changes in ultra violet visible (UV-VIS) spectral properties presented a decrease in typical color intensity and pointed towards formation of degradation products. Identification and kinetics of formation for cyanidin degradation products were obtained by high performance liquid chromatography system-mass spectrometry (HPLC-MS).
Keywords: HPLC-MS; UV-Vis; anthocyanins; ascorbic acid; kinetics; shelf life.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
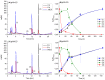
Figure 1
Stability of Cy and the formation of degradation products in buffered solutions stored at 37 °C: (A) a typical HPLC chromatogram at 270 nm of Cy solution (pH = 4.5) after 0, 6 and 146 h; (B) relative concentration (Ct/Cmax) of Cy and Cy degradation products (pH = 4.5); (C) a typical HPLC chromatogram at 270 nm of Cy solution (pH = 6.5) after 0, 6 and 146 h; and (D) relative concentration (Ct/Cmax) of Cy and Cy degradation products (pH = 6.5). Compounds identification: (1) 3,4-dihydroxybenzoic acid (Rt = 3.8); (2) 2,4,6-trihydroxybenzaldehyde (Rt = 11.9); (3) chalcone (Rt = 12.2); (4) Cy (Rt = 15.9); and (5) unidentified degradation product (Rt = 9.5). Quantification was made by HPLC-UV absorbance of the peak at 270 nm. Error bars represent standard error (n = 2). In some cases, they are smaller than the symbols. The lines in (B,D) are to guide the readers’ eye.
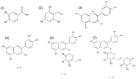
Figure 2
Chemical structure of anthocyanins and suggested degradation products: (1) 3,4-dihydroxybenzoic acid; (2) 2,4,6-trihydroxybenzaldehyde; (3) chalcone; (4) Cyanidin (Cy); (6) Cyanidin 3-O-β-glucoside (Cy3G); and (7) Cyanidin 3-O-β-rutinoside (Cy3R)). Structures were obtained using SciFinder® application.
Figure 3
Visual color of Cy stock solution and Cy in buffered solution pH = 4.5 and pH = 6.5, stored at 37 °C after 5 min, 6 h, 42 h and 143 h.
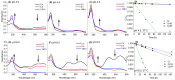
Figure 4
Stability (by HPLC) and changes in the absorbance spectrum of Cy, Cy3G and Cy3R stored at 37 °C: (A) average absorbance spectrum of Cy (pH = 4.5); (B) average absorbance spectrum of Cy3G (pH = 4.5); (C) average absorbance spectrum of Cy3R (pH = 4.5); (D) relative concentration (compared to t = 0) of Cy, Cy3G and Cy3R over time (pH = 4.5); (E) average absorbance spectrum of Cy (pH = 6.5); (F) average absorbance spectrum of Cy3G (pH = 6.5); (G) average absorbance spectrum of Cy3R (pH = 6.5); and (H) relative concentration (compared to t = 0) Cy, Cy3G and Cy3R over time (pH = 6.5). Quantification was made by HPLC-VIS absorbance of the peak at 516 nm and presented as percentage of the peak area divided by the initial peak area. (D,H) Error bars represent standard error (n = 2); in some cases, they are smaller than the symbols. The linear line represents fit to first-order degradation kinetics.
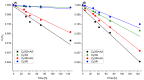
Figure 5
Stability of Cy3G and Cy3R in buffered solutions (pH 4.5 (A); and pH 6.5 (B)) over time with and without AA, stored at 37 °C. Quantification was made by HPLC absorbance of the peak at 516 nm and presented as relative concentration of the peak area divided by the initial peak area. Error bars represent standard error (n = 2). In most cases, they are smaller than the symbols.
Similar articles
- Effect on both aglycone and sugar moiety towards Phase II metabolism of anthocyanins.
Ichiyanagi T, Shida Y, Rahman MM, Sekiya M, Hatano Y, Matsumoto H, Hirayama M, Konishi T, Ikeshiro Y. Ichiyanagi T, et al. Food Chem. 2008 Sep 15;110(2):493-500. doi: 10.1016/j.foodchem.2008.02.031. Epub 2008 Feb 19. Food Chem. 2008. PMID: 26049244 - Influence of rutin and ascorbic acid in colour, plum anthocyanins and antioxidant capacity stability in model juices.
Hernández-Herrero JA, Frutos MJ. Hernández-Herrero JA, et al. Food Chem. 2015 Apr 15;173:495-500. doi: 10.1016/j.foodchem.2014.10.059. Epub 2014 Oct 18. Food Chem. 2015. PMID: 25466051 - Degradation of cyanidin 3-glucoside by caffeic acid o-quinone. Determination of the stoichiometry and characterization of the degradation products.
Kader F, Irmouli M, Zitouni N, Nicolas JP, Metche M. Kader F, et al. J Agric Food Chem. 1999 Nov;47(11):4625-30. doi: 10.1021/jf981400x. J Agric Food Chem. 1999. PMID: 10552861 - Red cabbage anthocyanins: Stability, extraction, biological activities and applications in food systems.
Ghareaghajlou N, Hallaj-Nezhadi S, Ghasempour Z. Ghareaghajlou N, et al. Food Chem. 2021 Dec 15;365:130482. doi: 10.1016/j.foodchem.2021.130482. Epub 2021 Jun 27. Food Chem. 2021. PMID: 34243124 Review. - Anthocyanin Profiling Using UV-Vis Spectroscopy and Liquid Chromatography Mass Spectrometry.
Saha S, Singh J, Paul A, Sarkar R, Khan Z, Banerjee K. Saha S, et al. J AOAC Int. 2020 Jan 1;103(1):23-39. doi: 10.5740/jaoacint.19-0201. J AOAC Int. 2020. PMID: 31462350 Review.
Cited by
- Complexation with Polysaccharides Enhances the Stability of Isolated Anthocyanins.
Fu W, Li S, Helmick H, Hamaker BR, Kokini JL, Reddivari L. Fu W, et al. Foods. 2023 Apr 29;12(9):1846. doi: 10.3390/foods12091846. Foods. 2023. PMID: 37174384 Free PMC article. - The Effect of Plant Additives on the Stability of Polyphenols in Cloudy and Clarified Juices from Black Chokeberry (Aronia melanocarpa).
Sidor A, Drożdżyńska A, Brzozowska A, Szwengiel A, Gramza-Michałowska A. Sidor A, et al. Antioxidants (Basel). 2020 Aug 27;9(9):801. doi: 10.3390/antiox9090801. Antioxidants (Basel). 2020. PMID: 32867376 Free PMC article. - Factors affecting the stability of anthocyanins and strategies for improving their stability: A review.
Xue H, Zhao J, Wang Y, Shi Z, Xie K, Liao X, Tan J. Xue H, et al. Food Chem X. 2024 Oct 5;24:101883. doi: 10.1016/j.fochx.2024.101883. eCollection 2024 Dec 30. Food Chem X. 2024. PMID: 39444439 Free PMC article. Review. - The Link between Polyphenol Structure, Antioxidant Capacity and Shelf-Life Stability in the Presence of Fructose and Ascorbic Acid.
Hanuka Katz I, Eran Nagar E, Okun Z, Shpigelman A. Hanuka Katz I, et al. Molecules. 2020 Jan 6;25(1):225. doi: 10.3390/molecules25010225. Molecules. 2020. PMID: 31935857 Free PMC article. - Anthocyanins: Factors Affecting Their Stability and Degradation.
Enaru B, Drețcanu G, Pop TD, Stǎnilǎ A, Diaconeasa Z. Enaru B, et al. Antioxidants (Basel). 2021 Dec 9;10(12):1967. doi: 10.3390/antiox10121967. Antioxidants (Basel). 2021. PMID: 34943070 Free PMC article. Review.
References
- Daravingas G., Cain R.F. Thermal degradation of black raspberry anthocyanin pigments in model systems. J. Food Sci. 1968;33:138–142. doi: 10.1111/j.1365-2621.1968.tb01338.x. - DOI
- Timberlake C.F. Anthocyanins-Occurrence, extraction and chemistry. Food Chem. 1980;5:69–80. doi: 10.1016/0308-8146(80)90065-5. - DOI
- Harborne J.B. Variation in and functional significance of phenolic conjugation in plants. In: Swain T., Harbone J.B., Van Sumere C.F., editors. Biochemistry of Plant Phenolics. Springer US; Boston, MA, USA: 2012. pp. 457–474.
- Jackman R.L., Yada R.Y., Tung M.A., Speers R.A. Anthocyanins as food colorants—A review. J. Food Biochem. 1987;11:201–247. doi: 10.1111/j.1745-4514.1987.tb00123.x. - DOI
LinkOut - more resources
Full Text Sources