Regulation of TEAD Transcription Factors in Cancer Biology - PubMed (original) (raw)
Review
Regulation of TEAD Transcription Factors in Cancer Biology
Hyunbin D Huh et al. Cells. 2019.
Abstract
Transcriptional enhanced associate domain (TEAD) transcription factors play important roles during development, cell proliferation, regeneration, and tissue homeostasis. TEAD integrates with and coordinates various signal transduction pathways including Hippo, Wnt, transforming growth factor beta (TGFβ), and epidermal growth factor receptor (EGFR) pathways. TEAD deregulation affects well-established cancer genes such as KRAS, BRAF, LKB1, NF2, and MYC, and its transcriptional output plays an important role in tumor progression, metastasis, cancer metabolism, immunity, and drug resistance. To date, TEADs have been recognized to be key transcription factors of the Hippo pathway. Therefore, most studies are focused on the Hippo kinases and YAP/TAZ, whereas the Hippo-dependent and Hippo-independent regulators and regulations governing TEAD only emerged recently. Deregulation of the TEAD transcriptional output plays important roles in tumor progression and serves as a prognostic biomarker due to high correlation with clinicopathological parameters in human malignancies. In addition, discovering the molecular mechanisms of TEAD, such as post-translational modifications and nucleocytoplasmic shuttling, represents an important means of modulating TEAD transcriptional activity. Collectively, this review highlights the role of TEAD in multistep-tumorigenesis by interacting with upstream oncogenic signaling pathways and controlling downstream target genes, which provides unprecedented insight and rationale into developing TEAD-targeted anticancer therapeutics.
Keywords: Hippo pathway; TEAD; cancer; stem cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
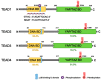
Figure 1
Domain architecture of human TEADs. The N-terminal DNA binding domain (DNA-BD) and C-terminal YAP/TAZ binding domain (YAP/TAZ-BD) of TEAD1-4 harbor high similarity across four different paralogs. The percent (%) represents the identity for each domain of TEADs compared to that of TEAD1 [50]. TEAD post-translation modifications include palmitoylation and PKA-, PKC-mediated phosphorylation that occur in the YAP/TAZ-BD and DNA-BD, respectively. Palmitoylation is required for proper TEAD functions. TEAD cytoplasmic translocation occurs through protein-protein interaction with p38 MAPK that binds the p38-binding motif within the DNA-BD of all TEADs.
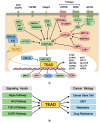
Figure 2
The regulatory mechanisms of TEAD in cancer biology. (a) Upstream signaling and downstream transcriptional outputs of TEAD. Various oncogenic signal transduction pathways, such as EGFR signaling, TGFβ signaling, Wnt signaling, GPCR signaling, and cancer genes (*), such as KRAS, BRAF, LKB1, APC, GNAQ/11 regulate TEAD activity through multiple signaling mechanisms. The TEAD transcriptional outputs have critical functions in tumorigenesis, stem cell maintenance, cancer immunology, metabolism as well as formation of signaling feedback loops. (b) Role of TEAD in multiple stages of tumorigenesis. TEAD activation via various oncogenic pathways play critical roles in cancer biology including EMT, metastasis, drug resistance, and cancer stem cells.
Similar articles
- Regulation of Hippo pathway transcription factor TEAD by p38 MAPK-induced cytoplasmic translocation.
Lin KC, Moroishi T, Meng Z, Jeong HS, Plouffe SW, Sekido Y, Han J, Park HW, Guan KL. Lin KC, et al. Nat Cell Biol. 2017 Jul 28;19(8):996-1002. doi: 10.1038/ncb3581. Nat Cell Biol. 2017. PMID: 28752853 Free PMC article. - Regulation of the Hippo Pathway Transcription Factor TEAD.
Lin KC, Park HW, Guan KL. Lin KC, et al. Trends Biochem Sci. 2017 Nov;42(11):862-872. doi: 10.1016/j.tibs.2017.09.003. Epub 2017 Sep 27. Trends Biochem Sci. 2017. PMID: 28964625 Free PMC article. Review. - The Hippo Tumor Suppressor Pathway (YAP/TAZ/TEAD/MST/LATS) and EGFR-RAS-RAF-MEK in cancer metastasis.
Zinatizadeh MR, Miri SR, Zarandi PK, Chalbatani GM, Rapôso C, Mirzaei HR, Akbari ME, Mahmoodzadeh H. Zinatizadeh MR, et al. Genes Dis. 2019 Dec 5;8(1):48-60. doi: 10.1016/j.gendis.2019.11.003. eCollection 2021 Jan. Genes Dis. 2019. PMID: 33569513 Free PMC article. Review. - A Novel Irreversible TEAD Inhibitor, SWTX-143, Blocks Hippo Pathway Transcriptional Output and Causes Tumor Regression in Preclinical Mesothelioma Models.
Hillen H, Candi A, Vanderhoydonck B, Kowalczyk W, Sansores-Garcia L, Kesikiadou EC, Van Huffel L, Spiessens L, Nijs M, Soons E, Haeck W, Klaassen H, Smets W, Spieser SA, Marchand A, Chaltin P, Ciesielski F, Debaene F, Chen L, Kamal A, Gwaltney SL, Versele M, Halder GA. Hillen H, et al. Mol Cancer Ther. 2024 Jan 3;23(1):3-13. doi: 10.1158/1535-7163.MCT-22-0681. Mol Cancer Ther. 2024. PMID: 37748190 - The TEAD Family and Its Oncogenic Role in Promoting Tumorigenesis.
Zhou Y, Huang T, Cheng AS, Yu J, Kang W, To KF. Zhou Y, et al. Int J Mol Sci. 2016 Jan 21;17(1):138. doi: 10.3390/ijms17010138. Int J Mol Sci. 2016. PMID: 26805820 Free PMC article. Review.
Cited by
- TEAD transcription factor family emerges as a promising therapeutic target for oral squamous cell carcinoma.
Wang S, Shao D, Gao X, Zhao P, Kong F, Deng J, Yang L, Shang W, Sun Y, Fu Z. Wang S, et al. Front Immunol. 2024 Oct 4;15:1480701. doi: 10.3389/fimmu.2024.1480701. eCollection 2024. Front Immunol. 2024. PMID: 39430767 Free PMC article. Review. - Long Non-Coding RNAs as Novel Targets for Phytochemicals to Cease Cancer Metastasis.
Rajabi S, Rajani HF, Mohammadkhani N, Ramírez-Coronel AA, Maleki M, Maresca M, Hajimehdipoor H. Rajabi S, et al. Molecules. 2023 Jan 18;28(3):987. doi: 10.3390/molecules28030987. Molecules. 2023. PMID: 36770654 Free PMC article. Review. - A novel HDAC11 inhibitor potentiates the tumoricidal effects of cordycepin against malignant peripheral nerve sheath tumor through the Hippo signaling pathway.
Huang PY, Shih IA, Liao YC, You HL, Lee MJ. Huang PY, et al. Am J Cancer Res. 2022 Feb 15;12(2):873-892. eCollection 2022. Am J Cancer Res. 2022. PMID: 35261809 Free PMC article. - The Hippo pathway drives the cellular response to hydrostatic pressure.
Park J, Jia S, Salter D, Bagnaninchi P, Hansen CG. Park J, et al. EMBO J. 2022 Jul 4;41(13):e108719. doi: 10.15252/embj.2021108719. Epub 2022 Jun 15. EMBO J. 2022. PMID: 35702882 Free PMC article. - Hippo pathway in non-small cell lung cancer: mechanisms, potential targets, and biomarkers.
Liang H, Xu Y, Zhao J, Chen M, Wang M. Liang H, et al. Cancer Gene Ther. 2024 May;31(5):652-666. doi: 10.1038/s41417-024-00761-z. Epub 2024 Mar 18. Cancer Gene Ther. 2024. PMID: 38499647 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous