Clinical Utility of Advanced Microbiology Testing Tools - PubMed (original) (raw)
Review
. 2019 Aug 26;57(9):e00495-19.
doi: 10.1128/JCM.00495-19. Print 2019 Sep.
Faranak Atrzadeh 2, C A Burnham 3, Stephen Cavalieri 4, James Dunn 5, Stephen Jones 6, Charles Mathews 7, Peggy McNult 8, John Meduri 9, Chris Newhouse 10, Duane Newton 11, Michael Oberholzer 12, John Osiecki 10, David Pedersen 6, Nicole Sweeney 13, Natalie Whitfield 14, Joe Campos; ASM Clinical and Public Health Microbiology Committee and the ASM Corporate Council
Affiliations
- PMID: 31217268
- PMCID: PMC6711927
- DOI: 10.1128/JCM.00495-19
Review
Clinical Utility of Advanced Microbiology Testing Tools
Melissa B Miller et al. J Clin Microbiol. 2019.
Abstract
Advanced microbiology technologies are rapidly changing our ability to diagnose infections, improve patient care, and enhance clinical workflow. These tools are increasing the breadth, depth, and speed of diagnostic data generated per patient, and testing is being moved closer to the patient through rapid diagnostic technologies, including point-of-care (POC) technologies. While select stakeholders have an appreciation of the value/importance of improvements in the microbial diagnostic field, there remains a disconnect between clinicians and some payers and hospital administrators in terms of understanding the potential clinical utility of these novel technologies. Therefore, a key challenge for the clinical microbiology community is to clearly articulate the value proposition of these technologies to encourage payers to cover and hospitals to adopt advanced microbiology tests. Specific guidance on how to define and demonstrate clinical utility would be valuable. Addressing this challenge will require alignment on this topic, not just by microbiologists but also by primary care and emergency room (ER) physicians, infectious disease specialists, pharmacists, hospital administrators, and government entities with an interest in public health. In this article, we discuss how to best conduct clinical studies to demonstrate and communicate clinical utility to payers and to set reasonable expectations for what diagnostic manufacturers should be required to demonstrate to support reimbursement from commercial payers and utilization by hospital systems.
Keywords: clinical utility; evidence; health economics; molecular methods; outcomes research; reimbursement.
Copyright © 2019 Miller et al.
Figures
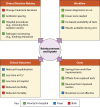
FIG 1
Endpoints for studies of clinical utility.
FIG 2
Select examples of types of trials that can be used to demonstrate clinical utility.
Similar articles
- Future of diagnostic microbiology.
Khardori N. Khardori N. Indian J Med Microbiol. 2014 Oct-Dec;32(4):371-7. doi: 10.4103/0255-0857.142233. Indian J Med Microbiol. 2014. PMID: 25297019 Review. - Practical issues in implementing whole-genome-sequencing in routine diagnostic microbiology.
Rossen JWA, Friedrich AW, Moran-Gilad J; ESCMID Study Group for Genomic and Molecular Diagnostics (ESGMD). Rossen JWA, et al. Clin Microbiol Infect. 2018 Apr;24(4):355-360. doi: 10.1016/j.cmi.2017.11.001. Epub 2017 Nov 5. Clin Microbiol Infect. 2018. PMID: 29117578 Review. - Better tests, better care: improved diagnostics for infectious diseases.
Caliendo AM, Gilbert DN, Ginocchio CC, Hanson KE, May L, Quinn TC, Tenover FC, Alland D, Blaschke AJ, Bonomo RA, Carroll KC, Ferraro MJ, Hirschhorn LR, Joseph WP, Karchmer T, MacIntyre AT, Reller LB, Jackson AF; Infectious Diseases Society of America (IDSA). Caliendo AM, et al. Clin Infect Dis. 2013 Dec;57 Suppl 3(Suppl 3):S139-70. doi: 10.1093/cid/cit578. Clin Infect Dis. 2013. PMID: 24200831 Free PMC article. Review. - The Brief Case: a New Feature in Journal of Clinical Microbiology.
Burnham CA, Onderdonk AB, McAdam AJ. Burnham CA, et al. J Clin Microbiol. 2016 Mar;54(3):512. doi: 10.1128/JCM.03269-15. Epub 2016 Jan 27. J Clin Microbiol. 2016. PMID: 26818673 Free PMC article. No abstract available. - Point-of-Care Testing in Microbiology.
Samuel L. Samuel L. Clin Lab Med. 2020 Dec;40(4):483-494. doi: 10.1016/j.cll.2020.08.006. Clin Lab Med. 2020. PMID: 33121617 Review.
Cited by
- 2020 American Society for Microbiology Awards Program Honorees in Clinical Microbiology.
Munson E. Munson E. J Clin Microbiol. 2020 Apr 23;58(5):e00082-20. doi: 10.1128/JCM.00082-20. Print 2020 Apr 23. J Clin Microbiol. 2020. PMID: 31996438 Free PMC article. No abstract available. - Prospective on Imaging Mass Spectrometry in Clinical Diagnostics.
Moore JL, Patterson NH, Norris JL, Caprioli RM. Moore JL, et al. Mol Cell Proteomics. 2023 Sep;22(9):100576. doi: 10.1016/j.mcpro.2023.100576. Epub 2023 May 19. Mol Cell Proteomics. 2023. PMID: 37209813 Free PMC article. Review. - The Impact of Polymerase Chain Reaction Urine Testing on Clinical Decision-Making in the Management of Complex Urinary Tract Infections.
Elia J, Hafron J, Holton M, Ervin C, Hollander MB, Kapoor DA. Elia J, et al. Int J Mol Sci. 2024 Jun 16;25(12):6616. doi: 10.3390/ijms25126616. Int J Mol Sci. 2024. PMID: 38928323 Free PMC article. - Understanding how diagnostics influence antimicrobial decision-making is key to successful clinical trial design.
Rawson TM, Moore LSP. Rawson TM, et al. Clin Microbiol Infect. 2023 Jun;29(6):666-669. doi: 10.1016/j.cmi.2023.03.010. Epub 2023 Mar 12. Clin Microbiol Infect. 2023. PMID: 36918143 Free PMC article. No abstract available. - Point-Counterpoint: Should We Be Performing Metagenomic Next-Generation Sequencing for Infectious Disease Diagnosis in the Clinical Laboratory?
Miller S, Chiu C, Rodino KG, Miller MB. Miller S, et al. J Clin Microbiol. 2020 Feb 24;58(3):e01739-19. doi: 10.1128/JCM.01739-19. Print 2020 Feb 24. J Clin Microbiol. 2020. PMID: 31619533 Free PMC article.
References
- Sousa AM, Pereira MO. 2013. A prospect of current microbial diagnosis methods, p 1429–1438. In Mendez-Vilas A. (ed), Microbial pathogens and strategies for combating them: science, technology and education, vol 3 Formatex, Bajadoz, Spain.
- Freeman K, Mistry H, Tsertsvadze A, Royle P, McCarthy N, Taylor-Phillips S, Manuel R, Mason J. 2017. Multiplex tests to identify gastrointestinal bacteria, viruses and parasites in people with suspected infectious gastroenteritis: a systematic review and economic analysis. Health Technol Assess 21. doi:10.3310/hta21230. - DOI - PMC - PubMed
- Centers for Disease Control and Prevention. 2017. Antibiotic use in the United States, 2017: progress and opportunities. Centers for Disease Control and Prevention, Atlanta, GA.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical