Reviewing evidence of the clinical effectiveness of commercially available antivenoms in sub-Saharan Africa identifies the need for a multi-centre, multi-antivenom clinical trial - PubMed (original) (raw)
Reviewing evidence of the clinical effectiveness of commercially available antivenoms in sub-Saharan Africa identifies the need for a multi-centre, multi-antivenom clinical trial
Julien Potet et al. PLoS Negl Trop Dis. 2019.
Abstract
Background: Snakebite envenoming kills more than more than 20,000 people in Sub-Saharan Africa every year. Poorly regulated markets have been inundated with low-price, low-quality antivenoms. This review aimed to systematically collect and analyse the clinical data on all antivenom products now available in markets of sub-Saharan Africa.
Methodology/principal findings: Our market analysis identified 12 polyspecific and 4 monospecific antivenom products in African markets. Our search strategy was first based on a systematic search of publication databases, followed by manual searches and discussions with experts. All types of data, including programmatic data, were eligible. All types of publications were eligible, including grey literature. Cohorts of less than 10 patients were excluded. 26 publications met the inclusion criteria. Many publications had to be excluded because clinical outcomes were not clearly linked to a specific product. Our narrative summaries present product-specific clinical data in terms of safety and effectiveness against the different species and envenoming syndromes. Three products (EchiTabPlus, EchiTabG, SAIMR-Echis-monovalent) were found to have been tested in robust clinical studies and found effective against envenoming caused by the West African carpet viper (Echis ocellatus). Four products (Inoserp-Panafricain, Fav-Afrique, SAIMR-Polyvalent, Antivipmyn-Africa) were found to have been evaluated only in observational single-arm studies, with varying results. For nine other products, there are either no data in the public domain, or only negative data suggesting a lack of effectiveness.
Conclusions/significance: Clinical data vary among the different antivenom products currently in African markets. Some products are available commercially although they have been found to lack effectiveness. The World Health Organization should strengthen its capacity to assess antivenom products, support antivenom manufacturers, and assist African countries and international aid organizations in selecting appropriate quality antivenoms.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
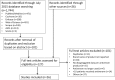
Fig 1. Search strategy.
Similar articles
- Pre-clinical assays predict pan-African Echis viper efficacy for a species-specific antivenom.
Casewell NR, Cook DA, Wagstaff SC, Nasidi A, Durfa N, Wüster W, Harrison RA. Casewell NR, et al. PLoS Negl Trop Dis. 2010 Oct 26;4(10):e851. doi: 10.1371/journal.pntd.0000851. PLoS Negl Trop Dis. 2010. PMID: 21049058 Free PMC article. - An analysis of preclinical efficacy testing of antivenoms for sub-Saharan Africa: Inadequate independent scrutiny and poor-quality reporting are barriers to improving snakebite treatment and management.
Ainsworth S, Menzies SK, Casewell NR, Harrison RA. Ainsworth S, et al. PLoS Negl Trop Dis. 2020 Aug 20;14(8):e0008579. doi: 10.1371/journal.pntd.0008579. eCollection 2020 Aug. PLoS Negl Trop Dis. 2020. PMID: 32817682 Free PMC article. - Antivenom therapy of carpet viper (Echis ocellatus) envenoming: effectiveness and strategies for delivery in West Africa.
Habib AG, Warrell DA. Habib AG, et al. Toxicon. 2013 Jul;69:82-9. doi: 10.1016/j.toxicon.2013.01.002. Epub 2013 Jan 20. Toxicon. 2013. PMID: 23339853 Review. - Antivenom for European Vipera species envenoming.
Lamb T, de Haro L, Lonati D, Brvar M, Eddleston M. Lamb T, et al. Clin Toxicol (Phila). 2017 Jul;55(6):557-568. doi: 10.1080/15563650.2017.1300261. Epub 2017 Mar 28. Clin Toxicol (Phila). 2017. PMID: 28349771 Review. - Evaluation of the preclinical efficacy of four antivenoms, distributed in sub-Saharan Africa, to neutralize the venom of the carpet viper, Echis ocellatus, from Mali, Cameroon, and Nigeria.
Sánchez LV, Pla D, Herrera M, Chippaux JP, Calvete JJ, Gutiérrez JM. Sánchez LV, et al. Toxicon. 2015 Nov;106:97-107. doi: 10.1016/j.toxicon.2015.09.027. Epub 2015 Sep 28. Toxicon. 2015. PMID: 26415904
Cited by
- Causes and Consequences of Snake Venom Variation.
Casewell NR, Jackson TNW, Laustsen AH, Sunagar K. Casewell NR, et al. Trends Pharmacol Sci. 2020 Aug;41(8):570-581. doi: 10.1016/j.tips.2020.05.006. Epub 2020 Jun 19. Trends Pharmacol Sci. 2020. PMID: 32564899 Free PMC article. Review. - Treatment outcomes among snakebite patients in north-west Ethiopia-A retrospective analysis.
Steegemans I, Sisay K, Nshimiyimana E, Gebrewold G, Piening T, Menberu Tessema E, Sahelie B, Alcoba G, Gebretsadik FS, Essink D, Collin S, Lucero E, Ritmeijer K. Steegemans I, et al. PLoS Negl Trop Dis. 2022 Feb 9;16(2):e0010148. doi: 10.1371/journal.pntd.0010148. eCollection 2022 Feb. PLoS Negl Trop Dis. 2022. PMID: 35139079 Free PMC article. - Interventions for the management of snakebite envenoming: An overview of systematic reviews.
Bhaumik S, Beri D, Lassi ZS, Jagnoor J. Bhaumik S, et al. PLoS Negl Trop Dis. 2020 Oct 13;14(10):e0008727. doi: 10.1371/journal.pntd.0008727. eCollection 2020 Oct. PLoS Negl Trop Dis. 2020. PMID: 33048936 Free PMC article. Review. - Terrestrial venomous animals, the envenomings they cause, and treatment perspectives in the Middle East and North Africa.
Jenkins TP, Ahmadi S, Bittenbinder MA, Stewart TK, Akgun DE, Hale M, Nasrabadi NN, Wolff DS, Vonk FJ, Kool J, Laustsen AH. Jenkins TP, et al. PLoS Negl Trop Dis. 2021 Dec 2;15(12):e0009880. doi: 10.1371/journal.pntd.0009880. eCollection 2021 Dec. PLoS Negl Trop Dis. 2021. PMID: 34855751 Free PMC article. Review. - 'The medicine is not for sale': Practices of traditional healers in snakebite envenoming in Ghana.
Steinhorst J, Aglanu LM, Ravensbergen SJ, Dari CD, Abass KM, Mireku SO, Adu Poku JK, Enuameh YAK, Blessmann J, Harrison RA, Amuasi JH, Stienstra Y. Steinhorst J, et al. PLoS Negl Trop Dis. 2021 Apr 16;15(4):e0009298. doi: 10.1371/journal.pntd.0009298. eCollection 2021 Apr. PLoS Negl Trop Dis. 2021. PMID: 33861735 Free PMC article.
References
- WHO (2010), Guidelines for the prevention and clinical management of snakebite in Africa. Brazzaville: World Health Organization
- Williams DJ., Faiz MA., Abela-Ridder B., Ainsworth S., Bulfone TC., Nickerson AD., et al. (2019) Strategy for a globally coordinated response to a priority neglected tropical disease: Snakebite envenoming. PLoS Neglected Tropical Diseases. Vol. 13(2): e0007059 10.1371/journal.pntd.0007059 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
The authors received no specific funding for this work.
LinkOut - more resources
Full Text Sources
Miscellaneous