Comparison of the macro and microstructure of sleep in a sample of sleep clinic hypersomnia cases - PubMed (original) (raw)
Comparison of the macro and microstructure of sleep in a sample of sleep clinic hypersomnia cases
Alyssa Cairns et al. Neurobiol Sleep Circadian Rhythms. 2019.
Abstract
The purpose of this study was to elucidate the differentiating or grouping EEG characteristics in various hypersomnias (type 1 and type 2 narcolepsy (N-1 and N-2) and idiopathic hypersomnia (IH) compared to an age-matched snoring reference group (SR). Polysomnogram sleep EEG was decomposed into a 4-frequency state model. The IH group had higher sleep efficiency (SE; 92.3% vs. 85.8%; sp < 0.05), lower WASO (IH = 35.4 vs. N-1 = 65.5 min; p < 0.01), but similar (i.e. high) arousal indices as N-1 (~33/h). N-1 and N-2 had earlier REM latency than IH and SR (N-1 = 64.8, N-2 = 76.3 vs. IH/SR = 118 min, p < 0.05). N-1 and N-2 showed an increase in MF1 segments (characteristic of stage 1 and REM) across the night as well as distinct oscillations every 2 h, but MF1 segment timing was advanced by 30 min compared to the SR group (p < 0.05). This suggests the presence of circadian organization to sleep that is timed earlier or of increased pressure and/or lability. MF1 demonstrated a mixed phenotype in IH, with an early 1st oscillation (like N-1 and N-2), 2nd oscillation that overlapped with the SR group, and a surge prior to wake (higher than all groups). This phenotype may reflect a heterogeneous group of individuals, with some having more narcolepsy-like characteristics (i.e. REM) than others. LF domain (delta surrogate) was enhanced in IH and N-1 and more rapidly dissipated compared to N-2 and SR (p < 0.05). This suggests an intact homeostatic sleep pattern that is of higher need/reduced efficiency whereas rapid dissipation may be an underlying mechanism for sleep disruption.
Keywords: MSLT; SOREMP; Sensitivity; Signal processing; Spectral analysis; Spectrum.
Figures
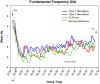
Fig. 1
Fundamental Frequency (Hz) Across time. Fundamental frequency = mean EEG cycles per second (Hz). Each vertical tick on the X-axis represents a 5-min time bin of the PSG (PSG = the first 7 h from Lights out; mean of 22:12 +/− 32 min). SO = mean sleep onset for all groups (no statistical differences between groups). Trend lines represent spline-smoothed changes in mean fundamental frequency over time. Bars represent standard error bars (SEM). Mixed model ANOVA with Bonferroni post-hoc comparisons was used to assess group (N = 4) by time (N = 82 time increments) changes. Main effect of time present with no interaction or group effects.
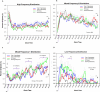
Fig. 2
Time-related changes in percent of epoch comprised of each respective EEG frequency segment across time. HF = characteristic of wakefulness, MF2 = characteristic of stage 2, MF1 = characteristic of stage 1 and REM; LF = characteristics of stage 3. Each vertical tick on the X-axis represents a 5-minute time bin of the PSG (PSG = the first 7 h from Lights out; mean of 22:12 +/− 32 min). SO = mean sleep onset for all groups (no statistical differences between groups). Trend lines represent spline-smoothed changes in mean percent of time comprised of each frequency component over time. Bars represent standard error bars (SEM). Mixed model ANOVA with Bonferroni post-hoc comparison to assess group (N = 4) by time (N = 82 time increments) changes. 2 A&B-main effect of time observed, no interaction or group effects. 2C- group by time interaction (p < 0.001); 1st oscillation- early in N-1, N-2 & IH compared to snoring controls p < .05), 2nd oscillation- N-1 & N-2 early vs. IH & snoring reference (p < 0.05); 3rd oscillation- N-1 & N-2 early vs. IH & snoring reference (p < 0.05). 2
d
- group by time interaction (p < 0.001); *a = N-1 & IH high LF domains than N-2 & snoring reference at acrophase (p < 0.05); *b = IH higher LF domains than all others (p < 0.05); precipitously decreasing at 1:00.
Similar articles
- Underutilization of the MSLT in sleepy patients with a short onset REM period (SOREMP) in the sleep clinic.
Cairns A, Bogan R. Cairns A, et al. Sleep Med. 2017 Apr;32:150-156. doi: 10.1016/j.sleep.2016.11.023. Epub 2017 Jan 21. Sleep Med. 2017. PMID: 28366327 - What Does One Sleep-Onset REM Period—During Either Nocturnal Polysomnography or Multiple Sleep Latency Test—Mean in Differential Diagnosis of Central Hypersomnias?
Bozluolcay M, Nalbantoglu M, Benbir Senel G, Karadeniz D. Bozluolcay M, et al. J Clin Neurophysiol. 2015 Aug;32(4):364-8. doi: 10.1097/WNP.0000000000000192. J Clin Neurophysiol. 2015. PMID: 26241245 - Different sleep onset criteria at the multiple sleep latency test (MSLT): an additional marker to differentiate central nervous system (CNS) hypersomnias.
Pizza F, Vandi S, Detto S, Poli F, Franceschini C, Montagna P, Plazzi G. Pizza F, et al. J Sleep Res. 2011 Mar;20(1 Pt 2):250-6. doi: 10.1111/j.1365-2869.2009.00808.x. J Sleep Res. 2011. PMID: 20337903 - The multiple sleep latency test.
Arand DL, Bonnet MH. Arand DL, et al. Handb Clin Neurol. 2019;160:393-403. doi: 10.1016/B978-0-444-64032-1.00026-6. Handb Clin Neurol. 2019. PMID: 31277864 Review. - Idiopathic hypersomnia.
Billiard M, Sonka K. Billiard M, et al. Sleep Med Rev. 2016 Oct;29:23-33. doi: 10.1016/j.smrv.2015.08.007. Epub 2015 Sep 3. Sleep Med Rev. 2016. PMID: 26599679 Review.
Cited by
- The Psychomotor Vigilance Test as a measure of alertness and sleep inertia in people with central disorders of hypersomnolence.
Trotti LM, Saini P, Bremer E, Mariano C, Moron D, Rye DB, Bliwise DL. Trotti LM, et al. J Clin Sleep Med. 2022 May 1;18(5):1395-1403. doi: 10.5664/jcsm.9884. J Clin Sleep Med. 2022. PMID: 35040431 Free PMC article. - Recognizing the Symptom Spectrum of Narcolepsy to Improve Timely Diagnosis: A Narrative Review.
Quaedackers L, Pillen S, Overeem S. Quaedackers L, et al. Nat Sci Sleep. 2021 Jul 7;13:1083-1096. doi: 10.2147/NSS.S278046. eCollection 2021. Nat Sci Sleep. 2021. PMID: 34262379 Free PMC article. Review. - Idiopathic Hypersomnia: Neurobiology, Diagnosis, and Management.
Morse AM, Naik S. Morse AM, et al. CNS Drugs. 2023 Apr;37(4):305-322. doi: 10.1007/s40263-023-00998-6. Epub 2023 Apr 18. CNS Drugs. 2023. PMID: 37069414 Review. - Diagnostic challenges and burden of idiopathic hypersomnia: a systematic literature review.
Boulanger T, Pigeon P, Crawford S. Boulanger T, et al. Sleep Adv. 2024 Aug 16;5(1):zpae059. doi: 10.1093/sleepadvances/zpae059. eCollection 2024. Sleep Adv. 2024. PMID: 39211350 Free PMC article.
References
- Achermann P. EEG analysis applies to sleep. Epilept. 2009;26:28–33.
- American Academy of Sleep Medicine . International Classification of Sleep Disorders. 3rd edn. American Academy of Sleep Medicine; Darien, IL: 2014.
LinkOut - more resources
Full Text Sources
Research Materials