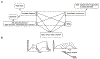Circadian Neurobiology and the Physiologic Regulation of Sleep and Wakefulness - PubMed (original) (raw)
Review
Circadian Neurobiology and the Physiologic Regulation of Sleep and Wakefulness
William J Schwartz et al. Neurol Clin. 2019 Aug.
Abstract
Endogenous central and peripheral circadian oscillators are key to organizing multiple aspects of mammalian physiology; this clock tracks the day-night cycle and governs behavioral and physiologic rhythmicity. Flexibility in the timing and duration of sleep and wakefulness, critical to the survival of species, is the result of a complex, dynamic interaction between 2 regulatory processes: the clock and a homeostatic drive that increases with wake duration and decreases during sleep. When circadian rhythmicity and sleep homeostasis are misaligned-as in shifted schedules, time zone transitions, aging, or disease-sleep, metabolic, and other disorders may ensue.
Keywords: Circadian rhythms; Homeostasis; Physiology; Sleep; Suprachiasmatic nucleus.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures
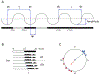
Figure 1.. Terminology and Displays
A. A schematic circadian rhythm, on the left entrained to a 12-h:12-h light:dark cycle and on the right free-running under constant darkness; white bar, light; black bar, dark. Under entrainment, zeitgeber time (zt) 0 represents the time of lights-on (dawn) and zt 12 the time of lights-off (dusk); during free-running, circadian time (ct) 0 represents “subjective” dawn and ct 12 “subjective” dusk. T = period length of the zeitgeber;τ = period length of the free-running rhythm; φ = phase, a defined, stable cycle-to-cycle reference point within the cycle of the rhythm (e.g.,φ1, the minimum value);Ψ = phase angle of entrainment, the time difference (phase relationship) between a defined phase of the rhythm and an external phase-reference (e.g., zt 0). B. A schematic circadian rhythm in “actogram” format. Data (e.g., locomotor activity) is plotted horizontally from left to right over the course of 24-h periods, with succeeding days stacked vertically from top to bottom. LD, light:dark cycle; DD, constant darkness. C. A schematic circular plot of the phases of individual rhythms from two populations, from 0 to 24 h (or sometimes graphed from 0 to 360°). The solid radial arrow represents the mean phase of each population and its length a measure of the scatter within each population. This format for plotting angular data illustrates that a mean of 15 h for the red population is not a larger value than a mean of 3 h for the blue population, but rather a difference in their relative phases.
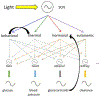
Figure 2.. A Network for Internal Time
Simplified cartoon of the mammalian circadian timekeeping system, with the suprachiasmatic nucleus (SCN) entrained by the light:dark cycle and “peripheral” body clocks entrained by SCN output signals, including rhythmic behaviors, body temperature, hormone levels, and nervous system activity; not shown are other clocks within the nervous system itself. These subsidiary clocks and rhythms also can respond to inputs downstream of the SCN (e.g., a shifted cycle of food availability on the hepatic clock). Of possible feedbacks, two exemplary ones are illustrated (i.e., locomotor activity itself affecting body temperature; adrenal output itself acting as a coupling signal).
Figure 3.. The Regulation of Sleep and Wake
A. Inter-relationships of factors affecting multiple aspects of sleep and wake. B. Diagrams of changes in time of (left) Two-Process Model of sleep regulation. S=Process S, the sleep homeostatic factor; C=Process C, the circadian rhythm process; Grey areas=time of sleep. When an individual self-selects their sleep onset (one of the two vertical lines), the level of homeostatic drive is at the Process S level (upper line); homeostatic drive then declines in an exponential manner until it reaches the Process C level. Then the person awakens and the level of homeostatic drive begins to rise again (which is not shown). (Right) Sleep durations associated with different self-selected sleep onsets.
Similar articles
- [Sleep and circadian rhythms].
Pickering L, Thorstensen EW, Riedel C, Jennum PJ. Pickering L, et al. Ugeskr Laeger. 2018 Sep 3;180(36):V05180319. Ugeskr Laeger. 2018. PMID: 30348254 Review. Danish. - Integration of human sleep-wake regulation and circadian rhythmicity.
Dijk DJ, Lockley SW. Dijk DJ, et al. J Appl Physiol (1985). 2002 Feb;92(2):852-62. doi: 10.1152/japplphysiol.00924.2001. J Appl Physiol (1985). 2002. PMID: 11796701 Review. - Circadian clock genes and sleep homeostasis.
Franken P, Dijk DJ. Franken P, et al. Eur J Neurosci. 2009 May;29(9):1820-9. doi: 10.1111/j.1460-9568.2009.06723.x. Epub 2009 Apr 28. Eur J Neurosci. 2009. PMID: 19473235 Review. - Suprachiasmatic nucleus in sleep-wake regulation.
Moore RY. Moore RY. Sleep Med. 2007 Dec;8 Suppl 3:27-33. doi: 10.1016/j.sleep.2007.10.003. Sleep Med. 2007. PMID: 18032104 Review. - [Circadian regulation of sleep-wake cycles and food anticipation].
Nakamura W. Nakamura W. Brain Nerve. 2012 Jun;64(6):647-56. Brain Nerve. 2012. PMID: 22647472 Review. Japanese.
Cited by
- Chronoradiobiology of Breast Cancer: The Time Is Now to Link Circadian Rhythm and Radiation Biology.
Nelson N, Lombardo J, Matlack L, Smith A, Hines K, Shi W, Simone NL. Nelson N, et al. Int J Mol Sci. 2022 Jan 25;23(3):1331. doi: 10.3390/ijms23031331. Int J Mol Sci. 2022. PMID: 35163264 Free PMC article. Review. - An Update on Prevalence, Assessment, and Risk Factors for Sleep Disturbances in Patients with Advanced Cancer-Implications for Health Care Providers and Clinical Research.
Jakobsen G, Gjeilo KH, Hjermstad MJ, Klepstad P. Jakobsen G, et al. Cancers (Basel). 2022 Aug 15;14(16):3933. doi: 10.3390/cancers14163933. Cancers (Basel). 2022. PMID: 36010925 Free PMC article. Review. - Sleep, Glial Function, and the Endocannabinoid System: Implications for Neuroinflammation and Sleep Disorders.
Camberos-Barraza J, Camacho-Zamora A, Bátiz-Beltrán JC, Osuna-Ramos JF, Rábago-Monzón ÁR, Valdez-Flores MA, Angulo-Rojo CE, Guadrón-Llanos AM, Picos-Cárdenas VJ, Calderón-Zamora L, Norzagaray-Valenzuela CD, Cárdenas-Torres FI, De la Herrán-Arita AK. Camberos-Barraza J, et al. Int J Mol Sci. 2024 Mar 9;25(6):3160. doi: 10.3390/ijms25063160. Int J Mol Sci. 2024. PMID: 38542134 Free PMC article. Review. - Light sensitivity of the circadian system in the social Highveld mole-rat Cryptomys hottentotus pretoriae.
Chanel PNC, Bennett NC, Oosthuizen MK. Chanel PNC, et al. J Exp Biol. 2024 Sep 15;227(18):jeb247793. doi: 10.1242/jeb.247793. Epub 2024 Sep 24. J Exp Biol. 2024. PMID: 39207238 Free PMC article. - REM-OSA as a Tool to Understand Both the Architecture of Sleep and Pathogenesis of Sleep Apnea-Literature Review.
Karuga FF, Kaczmarski P, Białasiewicz P, Szmyd B, Jaromirska J, Grzybowski F, Gebuza P, Sochal M, Gabryelska A. Karuga FF, et al. J Clin Med. 2023 Sep 12;12(18):5907. doi: 10.3390/jcm12185907. J Clin Med. 2023. PMID: 37762848 Free PMC article. Review.
References
- Dunlap JC, Loros JJ, DeCoursey PJ (eds). Chronobiology: Biological Timekeeping. Sunderland, MA: Sinauer Associates, 2009, 382 pp.
- Roenneberg T, Merrow M. The circadian clock and human health. Curr Biol 2016;26:R432–43. - PubMed
- Wever RA. Internal interactions within the human circadian system: the masking effect. Experientia 1985;41:332–42. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 HL128538/HL/NHLBI NIH HHS/United States
- K24 HL105664/HL/NHLBI NIH HHS/United States
- P01 AG009975/AG/NIA NIH HHS/United States
- UL1 TR001102/TR/NCATS NIH HHS/United States
- R01 GM105018/GM/NIGMS NIH HHS/United States
- R21 HD086392/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources