Role of the endolysosomal system in Parkinson's disease - PubMed (original) (raw)
Review
. 2019 Sep;150(5):487-506.
doi: 10.1111/jnc.14820. Epub 2019 Jul 31.
Affiliations
- PMID: 31287913
- PMCID: PMC6707858
- DOI: 10.1111/jnc.14820
Review
Role of the endolysosomal system in Parkinson's disease
D J Vidyadhara et al. J Neurochem. 2019 Sep.
Abstract
Parkinson's disease (PD) is one of the most common neurodegenerative disorders, affecting 1-1.5% of the total population. While progress has been made in understanding the neurodegenerative mechanisms that lead to cell death in late stages of PD, mechanisms for early, causal pathogenic events are still elusive. Recent developments in PD genetics increasingly point at endolysosomal (E-L) system dysfunction as the early pathomechanism and key pathway affected in PD. Clathrin-mediated synaptic endocytosis, an integral part of the neuronal E-L system, is probably the main early target as evident in auxilin, RME-8, and synaptojanin-1 mutations that cause PD. Autophagy, another important pathway in the E-L system, is crucial in maintaining proteostasis and a healthy mitochondrial pool, especially in neurons considering their inability to divide and requirement to function an entire life-time. PINK1 and Parkin mutations severely perturb autophagy of dysfunctional mitochondria (mitophagy), both in the cell body and synaptic terminals of dopaminergic neurons, leading to PD. Endolysosomal sorting and trafficking is also crucial, which is complex in multi-compartmentalized neurons. VPS35 and VPS13C mutations noted in PD target these mechanisms. Mutations in GBA comprise the most common risk factor for PD and initiate pathology by compromising lysosomal function. This is also the case for ATP13A2 mutations. Interestingly, α-synuclein and LRRK2, key proteins involved in PD, function in different steps of the E-L pathway and target their components to induce disease pathogenesis. In this review, we discuss these E-L system genes that are linked to PD and how their dysfunction results in PD pathogenesis. This article is part of the Special Issue "Synuclein".
Keywords: Autophagy; Clathrin-mediated endocytosis; LRRK2; Lysosomes; Retromer complex; α-Synuclein.
© 2019 International Society for Neurochemistry.
Conflict of interest statement
Conflict of interests: None
Figures
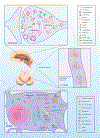
Figure 1. E-L system in the compartments of a nigrostriatal dopaminergic neuron, with the normal functional locations of E-L system proteins linked to PD.
A. Soma: E-L system proteins that form structural and functional components are synthesized in the neuronal soma. Following production in the endoplasmic reticulum (ER) and post-translational modifications in the Golgi apparatus, proteins (e.g. lysosomal hydrolases) are packed into vesicles and delivered to the trans-Golgi network (TGN). In the TGN, based on the nature of post-translational modification, the vesicles are diverted either towards lysosomes (black arrows) or to the secretory pathway (red arrows). Vesicles that are destined for lysosomes bud off from the TGN to form early endosomes. Simultaneously, early endosomes carrying nutrients and surface receptors for recycling are also derived from the plasma membrane through clathrin mediated endocytosis (CME, purple arrows). These early endosomes, with the help of the endosomal sorting complex required for transport (ESCRT), pass through highly dynamic tubulovesicular structures like multivesicular bodies (MVB) and/or late endosomes before fusing with lysosomes. Leucine-rich repeat kinase 2 (LRRK2) plays a significant role in this sorting, whereas Glucocerebrosidase 1 (GBA) and ATPase cation transporting 13A2 (ATP13A2) are crucial for normal lysosomal function. Early endosomes from the cell surface that are not sorted follow the recycling pathway back to the plasma membrane (blue arrows). Retrograde transport of receptors that are used for sorting from early endosomes to TGN occurs through the retromer complex where Vacuolar Protein Sorting 35 (VPS35) and VPS13C take part (yellow arrows). An expanding membrane sac called a phagophore sequesters malfunctioning organelles (e.g. sick mitochondria) and misfolded proteins to form a double-membraned autophagosomes. These later fuses with lysosomes and releases its contents for degradation and recycling of components; the complete process is called autophagy (green arrows). PTEN Induced Putative Kinase 1 (PINK1) and Parkin are important in identifying sick mitochondria and diverting them to a specialized form of autophagy called mitophagy. B. Axon: Axons form a crucial bridge for retrograde transport of endosomes/autophagosomes from presynaptic terminals to soma for lysosomal degradation. A gradual development of phagophore to full-fledged autophagosome happens within the axon (green arrows) as the encapsulated cargo arrives towards proximal end. C. Synapse: Neurotransmitter release in the presynaptic terminal is regulated principally by the synaptic vesicle cycle, an integral part of the neuronal E-L system. Initially, endosomes derived from the soma forms synaptic vesicles (SV) in the nerve terminal where RME-8 plays a role in association with clathrin (yellow arrow). Neurotransmitters are loaded into these SVs through specialized transporters. Upon arrival of an action potential in the synaptic terminal, these SVs fuse with the plasma membrane at active sites and release neurotransmitters via exocytosis (black arrows). Empty SVs are then recycled through CME (Purple arrows). α-Synuclein and endophilin A play roles here in membrane bending and SV curvature formation. Clathrin forms a layer on these invaginations with the help of adaptors, and in conjunction with dynamin leads to membrane fission forming clathrin coated vesicles (CCV). Clathrin uncoating is a prerequisite for SV recycling, which is performed by coordinated action of auxilin, Hsc70, endothelin A1, and synaptojanin 1. Phosphorylation by LRRK2 is necessary for activation of these proteins. Synaptic autophagy and mitophagy are also crucial in maintaining the health of the presynaptic terminal (green arrows), where PINK1/Parkin are crucial.
Similar articles
- LRRK2 and the Endolysosomal System in Parkinson's Disease.
Erb ML, Moore DJ. Erb ML, et al. J Parkinsons Dis. 2020;10(4):1271-1291. doi: 10.3233/JPD-202138. J Parkinsons Dis. 2020. PMID: 33044192 Free PMC article. Review. - Endosomal sorting pathways in the pathogenesis of Parkinson's disease.
Cunningham LA, Moore DJ. Cunningham LA, et al. Prog Brain Res. 2020;252:271-306. doi: 10.1016/bs.pbr.2020.02.001. Epub 2020 Mar 16. Prog Brain Res. 2020. PMID: 32247367 Free PMC article. Review. - Synaptic, Mitochondrial, and Lysosomal Dysfunction in Parkinson's Disease.
Nguyen M, Wong YC, Ysselstein D, Severino A, Krainc D. Nguyen M, et al. Trends Neurosci. 2019 Feb;42(2):140-149. doi: 10.1016/j.tins.2018.11.001. Epub 2018 Nov 30. Trends Neurosci. 2019. PMID: 30509690 Free PMC article. Review. - Genetic Evidence for Endolysosomal Dysfunction in Parkinson's Disease: A Critical Overview.
Yahya V, Di Fonzo A, Monfrini E. Yahya V, et al. Int J Mol Sci. 2023 Mar 28;24(7):6338. doi: 10.3390/ijms24076338. Int J Mol Sci. 2023. PMID: 37047309 Free PMC article. Review. - Alpha-Synuclein and the Endolysosomal System in Parkinson's Disease: Guilty by Association.
Teixeira M, Sheta R, Idi W, Oueslati A. Teixeira M, et al. Biomolecules. 2021 Sep 9;11(9):1333. doi: 10.3390/biom11091333. Biomolecules. 2021. PMID: 34572546 Free PMC article. Review.
Cited by
- Methamphetamine Increases Tubulo-Vesicular Areas While Dissipating Proteins from Vesicles Involved in Cell Clearance.
Lazzeri G, Lenzi P, Busceti CL, Puglisi-Allegra S, Ferrucci M, Fornai F. Lazzeri G, et al. Int J Mol Sci. 2024 Sep 4;25(17):9601. doi: 10.3390/ijms25179601. Int J Mol Sci. 2024. PMID: 39273545 Free PMC article. - Inter-organellar Communication in Parkinson's and Alzheimer's Disease: Looking Beyond Endoplasmic Reticulum-Mitochondria Contact Sites.
Vrijsen S, Vrancx C, Del Vecchio M, Swinnen JV, Agostinis P, Winderickx J, Vangheluwe P, Annaert W. Vrijsen S, et al. Front Neurosci. 2022 Jun 21;16:900338. doi: 10.3389/fnins.2022.900338. eCollection 2022. Front Neurosci. 2022. PMID: 35801175 Free PMC article. - Dopaminergic Axons: Key Recitalists in Parkinson's Disease.
Mishra AK, Dixit A. Mishra AK, et al. Neurochem Res. 2022 Feb;47(2):234-248. doi: 10.1007/s11064-021-03464-1. Epub 2021 Oct 12. Neurochem Res. 2022. PMID: 34637100 Review. - Plasma acellular transcriptome contains Parkinson's disease signatures that can inform clinical diagnosis.
Beric A, Cisterna-García A, Martin C, Kumar R, Alfradique-Dunham I, Boyer K, Saliu IO, Yamada S, Sanford J, Western D, Liu M, Alvarez I, Perlmutter JS, Norris SA, Pastor P, Zhao G, Botia J, Ibanez L. Beric A, et al. medRxiv [Preprint]. 2024 Oct 18:2024.10.18.24315717. doi: 10.1101/2024.10.18.24315717. medRxiv. 2024. PMID: 39484251 Free PMC article. Preprint. - Synaptic vesicle endocytosis deficits underlie GBA-linked cognitive dysfunction in Parkinson's disease and Dementia with Lewy bodies.
Vidyadhara DJ, Bäckström D, Chakraborty R, Ruan J, Park JM, Mistry PK, Chandra SS. Vidyadhara DJ, et al. bioRxiv [Preprint]. 2025 Jan 2:2024.10.23.619548. doi: 10.1101/2024.10.23.619548. bioRxiv. 2025. PMID: 39484386 Free PMC article. Preprint.
References
- Aharon-Peretz J, Rosenbaum H and Gershoni-Baruch R (2004) Mutations in the glucocerebrosidase gene and Parkinson’s disease in Ashkenazi Jews. N Engl J Med 351, 1972–1977. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS083846/NS/NINDS NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- RF1 NS110354/NS/NINDS NIH HHS/United States
- R01 NS064963/NS/NINDS NIH HHS/United States
- R01 NS110354/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous