Arthritis prevention in the pre-clinical phase of RA with abatacept (the APIPPRA study): a multi-centre, randomised, double-blind, parallel-group, placebo-controlled clinical trial protocol - PubMed (original) (raw)
doi: 10.1186/s13063-019-3403-7.
Marianna Jasenecova 2, Sonya Abraham 3, Aisla Bosworth 4, Ian N Bruce 5 6, Christopher D Buckley 7 8, Coziana Ciurtin 9, Maria-Antonietta D'Agostino 10 11, Paul Emery 12, Hill Gaston 13, John D Isaacs 14 15, Andrew Filer 7 8, Benjamin A Fisher 7 8, Thomas W J Huizinga 16, Pauline Ho 5 6, Clare Jacklin 4, Heidi Lempp 2, Iain B McInnes 17, Arthur G Pratt 14 15, Andrew Östor 13, Karim Raza 7 8, Peter C Taylor 18, Dirkjan van Schaardenburg 19, Dharshene Shivapatham 2, Alison J Wright 20, Joana C Vasconcelos 21, Joanna Kelly 22, Caroline Murphy 22, A Toby Prevost 21, Andrew P Cope 23
Affiliations
- PMID: 31307535
- PMCID: PMC6633323
- DOI: 10.1186/s13063-019-3403-7
Arthritis prevention in the pre-clinical phase of RA with abatacept (the APIPPRA study): a multi-centre, randomised, double-blind, parallel-group, placebo-controlled clinical trial protocol
Mariam Al-Laith et al. Trials. 2019.
Abstract
Trial design: We present a study protocol for a multi-centre, randomised, double-blind, parallel-group, placebo-controlled trial that seeks to test the feasibility, acceptability and effectiveness of a 52-week period of treatment with the first-in-class co-stimulatory blocker abatacept for preventing or delaying the onset of inflammatory arthritis.
Methods: The study aimed to recruit 206 male or female subjects from the secondary care hospital setting across the UK and the Netherlands. Participants who were at least 18 years old, who reported inflammatory sounding joint pain (clinically suspicious arthralgia) and who were found to be positive for serum autoantibodies associated with rheumatoid arthritis (RA) were eligible for enrolment. All study subjects were randomly assigned to receive weekly injections of investigational medicinal product, either abatacept or placebo treatment over the course of a 52-week period. Participants were followed up for a further 52 weeks. The primary endpoint was defined as the time to development of at least three swollen joints or to the fulfilment of the 2010 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) classification criteria for RA using swollen but not tender joints, whichever endpoint was met first. In either case, swollen joints were confirmed by ultrasonography. Participants, care givers, and those assessing the outcomes were all blinded to group assignment. Clinical assessors and ultrasonographers were also blinded to each other's assessments for the duration of the study.
Conclusions: There is limited experience of the design and implementation of trials for the prevention of inflammatory joint diseases. We discuss the rationale behind choice and duration of treatment and the challenges associated with defining the "at risk" state and offer pragmatic solutions in the protocol to enrolling subjects at risk of RA.
Trial registration: Current Controlled Trials, ID: ISRCTN46017566 . Registered on 4 July 2014.
Keywords: Abatacept; Antibodies to citrullinated protein antigens; At risk; Autoantibodies; Double-blind; Intervention; Placebo-controlled; Pre-clinical phase; Randomised; Rheumatoid arthritis.
Conflict of interest statement
MA-L, MJ, SA, AB, CC, HG, AF, BAF, PH, CJ, HL, DvS, DS, AJW, JCV, JK, CM and ATP declare that they have no competing interests relating to this study. INB, CDB, M-AD’A, PE, TWJH, JDI, IBM, AÖ, AGP, KR, PCT, and APC have received honoraria, speaker fees or research funding (or a combination of these) from Bristol-Myers Squibb or from other pharmaceutical companies.
Figures
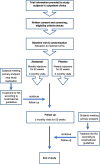
Fig. 1
Study design and flow chart
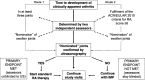
Fig. 2
Primary endpoint roadmap
Similar articles
- Abatacept in individuals at high risk of rheumatoid arthritis (APIPPRA): a randomised, double-blind, multicentre, parallel, placebo-controlled, phase 2b clinical trial.
Cope AP, Jasenecova M, Vasconcelos JC, Filer A, Raza K, Qureshi S, D'Agostino MA, McInnes IB, Isaacs JD, Pratt AG, Fisher BA, Buckley CD, Emery P, Ho P, Buch MH, Ciurtin C, van Schaardenburg D, Huizinga T, Toes R, Georgiou E, Kelly J, Murphy C, Prevost AT; APIPPRA study investigators. Cope AP, et al. Lancet. 2024 Mar 2;403(10429):838-849. doi: 10.1016/S0140-6736(23)02649-1. Epub 2024 Feb 13. Lancet. 2024. PMID: 38364839 Clinical Trial. - TREAT Early Arthralgia to Reverse or Limit Impending Exacerbation to Rheumatoid arthritis (TREAT EARLIER): a randomized, double-blind, placebo-controlled clinical trial protocol.
Niemantsverdriet E, Dakkak YJ, Burgers LE, Bonte-Mineur F, Steup-Beekman GM, van der Kooij SM, Boom HD, Allaart CF, de Jong PHP, van der Helm-van Mil AHM. Niemantsverdriet E, et al. Trials. 2020 Oct 16;21(1):862. doi: 10.1186/s13063-020-04731-2. Trials. 2020. PMID: 33076964 Free PMC article. - Abatacept inhibits inflammation and onset of rheumatoid arthritis in individuals at high risk (ARIAA): a randomised, international, multicentre, double-blind, placebo-controlled trial.
Rech J, Tascilar K, Hagen M, Kleyer A, Manger B, Schoenau V, Hueber AJ, Kleinert S, Baraliakos X, Braun J, Kiltz U, Fleck M, Rubbert-Roth A, Kofler DM, Behrens F, Feuchtenberger M, Zaenker M, Voll R, Venhoff N, Thiel J, Glaser C, Feist E, Burmester GR, Karberg K, Strunk J, Cañete JD, Senolt L, Filkova M, Naredo E, Largo R, Krönke G, D'Agostino MA, Østergaard M, Schett G. Rech J, et al. Lancet. 2024 Mar 2;403(10429):850-859. doi: 10.1016/S0140-6736(23)02650-8. Epub 2024 Feb 13. Lancet. 2024. PMID: 38364841 Clinical Trial. - Balneotherapy (or spa therapy) for rheumatoid arthritis.
Verhagen AP, Bierma-Zeinstra SM, Boers M, Cardoso JR, Lambeck J, de Bie R, de Vet HC. Verhagen AP, et al. Cochrane Database Syst Rev. 2015 Apr 11;2015(4):CD000518. doi: 10.1002/14651858.CD000518.pub2. Cochrane Database Syst Rev. 2015. PMID: 25862243 Free PMC article. Review. - Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
Cited by
- Patients' and rheumatologists' perceptions on preventive intervention in rheumatoid arthritis and axial spondyloarthritis.
van Boheemen L, Bolt JW, Ter Wee MM, de Jong HM, van de Sande MG, van Schaardenburg D. van Boheemen L, et al. Arthritis Res Ther. 2020 Sep 15;22(1):217. doi: 10.1186/s13075-020-02314-9. Arthritis Res Ther. 2020. PMID: 32933547 Free PMC article. - Efficacy of guselkumab, a selective IL-23 inhibitor, in Preventing Arthritis in a Multicentre Psoriasis At-Risk cohort (PAMPA): protocol of a randomised, double-blind, placebo controlled multicentre trial.
Haberman RH, MacFarlane KA, Catron S, Samuels J, Blank RB, Toprover M, Uddin Z, Hu J, Castillo R, Gong C, Qian K, Piguet V, Tausk F, Yeung J, Neimann AL, Gulliver W, Thiele RG, Merola JF, Ogdie A, Rahman P, Chakravarty SD, Eder L, Ritchlin CT, Scher JU. Haberman RH, et al. BMJ Open. 2022 Dec 23;12(12):e063650. doi: 10.1136/bmjopen-2022-063650. BMJ Open. 2022. PMID: 36564123 Free PMC article. - A Roadmap for Investigating Preclinical Autoimmunity Using Patient-Oriented and Epidemiologic Study Designs: Example of Rheumatoid Arthritis.
Kowalski EN, Qian G, Vanni KMM, Sparks JA. Kowalski EN, et al. Front Immunol. 2022 May 25;13:890996. doi: 10.3389/fimmu.2022.890996. eCollection 2022. Front Immunol. 2022. PMID: 35693829 Free PMC article. Review. - Rheumatoid arthritis prevention in arthralgia: fantasy or reality?
van Steenbergen HW, Cope AP, van der Helm-van Mil AHM. van Steenbergen HW, et al. Nat Rev Rheumatol. 2023 Dec;19(12):767-777. doi: 10.1038/s41584-023-01035-y. Epub 2023 Oct 9. Nat Rev Rheumatol. 2023. PMID: 37814057 Review. - Preclinical rheumatoid arthritis and rheumatoid arthritis prevention.
Greenblatt HK, Kim HA, Bettner LF, Deane KD. Greenblatt HK, et al. Curr Opin Rheumatol. 2020 May;32(3):289-296. doi: 10.1097/BOR.0000000000000708. Curr Opin Rheumatol. 2020. PMID: 32205569 Free PMC article. Review.
References
- Wolfe F, Hawley DJ. The longterm outcomes of rheumatoid arthritis: Work disability: a prospective 18 year study of 823 patients. J Rheumatol. 1998;25:2108–2117. - PubMed
- Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, van Zeben D, Kerstens PJ, Hazes JM, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum. 2005;52:3381–3390. doi: 10.1002/art.21405. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous