Chemopreventive Effect of Phytosomal Curcumin on Hepatitis B Virus-Related Hepatocellular Carcinoma in A Transgenic Mouse Model - PubMed (original) (raw)
Chemopreventive Effect of Phytosomal Curcumin on Hepatitis B Virus-Related Hepatocellular Carcinoma in A Transgenic Mouse Model
Chiao-Fang Teng et al. Sci Rep. 2019.
Abstract
Chronic hepatitis B virus (HBV) infection is a major risk factor for the development of hepatocellular carcinoma (HCC), a leading cause of cancer mortality worldwide. Hepatitis B X protein (HBx) and pre-S2 mutant have been proposed as the two most important HBV oncoproteins that play key roles in HCC pathogenesis. Curcumin is a botanical constituent displaying potent anti-inflammatory and anti-cancer properties without toxic side effects. Phytosomal formulation of curcumin has been shown to exhibit enhanced bioavailability, improved pharmacokinetics, and excellent efficacy against many human diseases. However, effectiveness of phytosomal curcumin for HCC treatment remains to be clarified. In this study, we evaluated chemopreventive effect of phytosomal curcumin on HBV-related HCC by using a transgenic mouse model specifically expressing both HBx and pre-S2 mutant in liver. Compared with unformulated curcumin, phytosomal curcumin exhibited significantly greater effects on suppression of HCC formation, improvement of liver histopathology, decrease of lipid accumulation and leukocyte infiltration, and reduction of total tumor volume in transgenic mice. Moreover, phytosomal curcumin exerted considerably stronger effects on activation of anti-inflammatory PPARγ as well as inhibition of pro-inflammatory NF-κB than unformulated curcumin. Furthermore, phytosomal curcumin showed a comparable effect on suppression of oncogenic mTOR activation to unformulated curcumin. Our data demonstrated that phytosomal curcumin has promise for HCC chemoprevention in patients with chronic HBV infection.
Conflict of interest statement
The authors declare no competing interests.
Figures
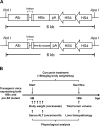
Figure 1
Establishment and curcumin treatment of the transgenic mouse model expressing both HBx and pre-S2 mutant. (A) Schematic diagram of the albumin-HBx and -pre-S2 mutant transgenic constructs. The HBx and pre-S2 mutant transgenes were driven by the liver-specific albumin promoter. The NotI and ApaI restriction enzyme sites were used to excise the 6-kb DNA insert for pronucleus microinjection. (B) Schematic diagram of curcumin treatment. The transgenic mice were treated with either normal diets or the diets containing unformulated curcumin or phytosomal curcumin (150 mg curcuminoids/kg body weight/day), beginning at 9 months of age, for 6 months, and were then sacrificed. The body weight and serum ALT of mice were measured immediately before treatment and routinely after treatment until sacrifice. At sacrifice, the total tumor volume of mice was measured and the histopathology of mice liver was examined.
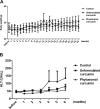
Figure 2
Phytosomal curcumin did not affect body weight and lowered serum ALT level in transgenic mice expressing both HBx and pre-S2 mutant. The body weight (A) and serum ALT level (B) of the transgenic mice were measured immediately before treatment and once a week or month after treatment of normal diets (control), unformulated or phytosomal curcumin diets for 6 consecutive months. Data represent the mean with standard error of the mean (SEM) error bar.
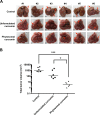
Figure 3
Phytosomal curcumin inhibited HCC formation and reduced the total tumor volume in liver of transgenic mice expressing both HBx and pre-S2 mutant. (A) The formation of tumor in the transgenic mice liver was examined at the end of treatment of normal diets (control), unformulated or phytosomal curcumin diets. Tumors were encircled by red dashed lines and indicated by red arrows. Scale bar, 5 mm. (B) The total tumor volume in the transgenic mice liver from each treatment group was determined. The y axis is on the log scale. Horizontal lines represent the median values of the distribution. A statistical significance of the difference between the control mice and phytosomal curcumin diets-treated mice rather than the unformulated curcumin diets-treated mice was shown. A statistical significance of the difference between the phytosomal curcumin diets- and unformulated curcumin diets-treated mice was also observed. *P < 0.05, ***P < 0.001.
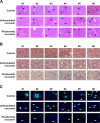
Figure 4
Phytosomal curcumin improved histopathology and decreased lipid accumulation and leukocyte infiltration in liver of transgenic mice expressing both HBx and pre-S2 mutant. (A) The histopathology of the transgenic mice liver was examined at the end of treatment of normal diets (control), unformulated or phytosomal curcumin diets by H&E staining. Black arrows indicate the steatosis (small lipid droplets) and black arrowheads indicate the necroinflammation (inflammatory cell clusters). Shown were representative results of each mouse. Original magnification, ×20. Scale bar, 100 μm. (B) The intracellular lipid deposit (red in color) in liver of each treatment group of mice was evaluated by Oil Red O staining. (C) The expression of CD45 (green in color) in liver tissues of each treatment group of mice was detected by fluorescent IHC staining. Nuclei were stained with DAPI (blue in color). White arrows and white dashed circles indicate the single and clustered CD45-positive cells, respectively. Shown were representative results of each mouse. Original magnification, ×40. Scale bar, 50 μm.
Figure 5
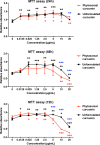
Phytosomal curcumin suppressed the growth of HCC cells. HuH-7 cells were either left untreated or treated with unformulated or phytosomal curcumin with the indicated working concentrations for 24, 48, and 72 hours. At the indicated time points, the level of cell growth in each treatment was determined by MTT assay for measuring the absorbance values at 450 nm. Data represent the mean relative to the untreated cells from three independent experiments. Error bars indicate SD. Black and red asterisks denote the statistically significant differences between the untreated cells and either unformulated or phytosomal curcumin-treated cells, respectively. The statistically significant differences between the unformulated and phytosomal curcumin-treated cells were further indicated by blue asterisks. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 6
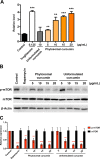
Phytosomal curcumin enhanced PPARγ activity and suppressed mTOR activation in HCC cells. (A) HuH-7 cells were transfected with a GAL4-driven reporter plasmid and a plasmid encoding the PPARγ LBD fused with the GAL4 DNA-binding domain. After transfection, the cells were either left untreated (control) or treated with the indicated working concentrations of troglitazone, unformulated curcumin, or phytosomal curcumin. The activity of PPARγ in the cells was measured by luciferase reporter assay. Data represent the mean relative to the control cells. Error bars indicate SD. Black asterisks denote the statistically significant differences between the treated and control cells. **P < 0.01, ***P < 0.001. (B) HuH-7 cells were either left untreated (control) or treated with a working concentration of 200 nM of rapamycin or with the indicated working concentrations of unformulated or phytosomal curcumin. After treatment, the expression of phosphorylated (p) activated form of mTOR was detected by Western blot analysis. Shown were representative results from three independent experiments. (C) Quantitative and statistical analysis of the Western blotting data. Data in each experiment were presented as mean values relative to the control cells. Error bars indicate SD. Red asterisks denote the statistically significant differences between the treated and control cells. **P < 0.01, ***P < 0.001.
Figure 7
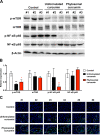
Phytosomal curcumin activated PPARγ but inhibited both NF-κB and mTOR activity in liver of transgenic mice expressing both HBx and pre-S2 mutant. (A) Liver tissues were isolated from the transgenic mice at the end of treatment of normal diets (control), unformulated or phytosomal curcumin diets. The expression of phosphorylated (p) activated form of NF-κB p65 and mTOR was detected by Western blot analysis. Three livers were used in each treatment group. (B) Quantitative and statistical analysis of the Western blotting data. Data in each treatment group of mice were presented as mean values relative to the control mice. Error bars indicate SD. Black and red asterisks denote the statistically significant differences between the control mice and either unformulated or phytosomal curcumin diets-treated mice, respectively. *P < 0.05, **P < 0.01. (C) Detection of nuclear PPARγ expression in liver tissues of each treatment group of mice by fluorescent IHC staining. Localization of PPARγ (green in color) in the nucleus (blue in color) appeared cyan and was highlighted by white dashed circles. Shown were representative results of each mouse. Original magnification, ×40. Scale bar, 50 μm.
Figure 8
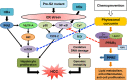
Schematic model for the chemopreventive effect of phytosomal curcumin on HBV-related HCC. In chronic HBV infection, two HBV oncoproteins, HBx and pre-S2 mutant, play key roles in the progression of HCC through either the induction of ER stress-induced oxidative DNA damage to cause genomic instability or the activation of NF-κB and mTOR signal pathways to promote hepatocyte proliferation. Phytosomal curcumin may exert its chemopreventive effects on HBV-related HCC through three mechanisms: one involving the activation of PPARγ activity to upregulate the expression of genes involved in lipid metabolism, anti-inflammation, and anti-cell proliferation (as indicated by sequential orange arrows), another involving the inhibition of NF-κB activation though PPARγ to repress the expression of pro-inflammatory cytokines (as indicated by red dashed lines), and the other involving the suppression of mTOR activation to block hepatocyte proliferation (as indicated by blue dashed lines). Abbreviations: HBV, hepatitis B virus; HBx, hepatitis B X protein; HCC, hepatocellular carcinoma; ER, endoplasmic reticulum; NF-κB, nuclear factor-κB; mTOR, mammalian target of rapamycin; PPARγ, peroxisome proliferator-activated reporter γ; RXR, retinoid X receptor; PPRE, peroxisome proliferator response element; VEGF-A, vascular endothelial growth factor-A; COX-2, cyclooxygenase-2; IKKβ, IκB kinase β; Ca2+, ionized calcium; ROI, reactive oxygen intermediates.
Similar articles
- Hepatitis B virus pre-S2 mutant large surface protein inhibits DNA double-strand break repair and leads to genome instability in hepatocarcinogenesis.
Hsieh YH, Chang YY, Su IJ, Yen CJ, Liu YR, Liu RJ, Hsieh WC, Tsai HW, Wang LH, Huang W. Hsieh YH, et al. J Pathol. 2015 Jul;236(3):337-47. doi: 10.1002/path.4531. Epub 2015 Apr 22. J Pathol. 2015. PMID: 25775999 - Expression of a hepatitis B virus pre-S2 deletion mutant in the liver results in hepatomegaly and hepatocellular carcinoma in mice.
Teng YC, Neo JC, Wu JC, Chen YF, Kao CH, Tsai TF. Teng YC, et al. J Pathol. 2017 Mar;241(4):463-474. doi: 10.1002/path.4850. Epub 2017 Jan 5. J Pathol. 2017. PMID: 27868197 - Histone deacetylase inhibitor suberoylanilide hydroxamic acid suppresses the pro-oncogenic effects induced by hepatitis B virus pre-S2 mutant oncoprotein and represents a potential chemopreventive agent in high-risk chronic HBV patients.
Hsieh YH, Su IJ, Yen CJ, Tsai TF, Tsai HW, Tsai HN, Huang YJ, Chen YY, Ai YL, Kao LY, Hsieh WC, Wu HC, Huang W. Hsieh YH, et al. Carcinogenesis. 2013 Feb;34(2):475-85. doi: 10.1093/carcin/bgs365. Epub 2012 Nov 21. Carcinogenesis. 2013. PMID: 23172669 - Hepatitis B virus pre-S/S variants in liver diseases.
Chen BF. Chen BF. World J Gastroenterol. 2018 Apr 14;24(14):1507-1520. doi: 10.3748/wjg.v24.i14.1507. World J Gastroenterol. 2018. PMID: 29662289 Free PMC article. Review. - Molecular mechanistic insight of hepatitis B virus mediated hepatocellular carcinoma.
Chaturvedi VK, Singh A, Dubey SK, Hetta HF, John J, Singh MP. Chaturvedi VK, et al. Microb Pathog. 2019 Mar;128:184-194. doi: 10.1016/j.micpath.2019.01.004. Epub 2019 Jan 3. Microb Pathog. 2019. PMID: 30611768 Review.
Cited by
- A Presurgical Study of Curcumin Combined with Anthocyanin Supplements in Patients with Colorectal Adenomatous Polyps.
Briata IM, Paleari L, Rutigliani M, Petrera M, Caviglia S, Romagnoli P, Libera MD, Oppezzi M, Puntoni M, Siri G, Lazzeroni M, Howells L, Singh R, Brown K, DeCensi A. Briata IM, et al. Int J Mol Sci. 2021 Oct 13;22(20):11024. doi: 10.3390/ijms222011024. Int J Mol Sci. 2021. PMID: 34681684 Free PMC article. - Exploring new therapeutic potentials of curcumin against post-surgical adhesion bands.
Askarnia-Faal MM, Sayyed-Hosseinian SH, Nazari SE, Asgharzadeh F, Vahedi E, Eskandari M, Ghasemi H, Avan A, Alaei M, Naimi H, Daghiani M, Soleimani A, Alalikhan A, Mohammadzadeh R, Ferns G, Ryzhikov M, Khazaei M, Hassanian SM. Askarnia-Faal MM, et al. BMC Complement Med Ther. 2023 Jan 31;23(1):27. doi: 10.1186/s12906-022-03808-6. BMC Complement Med Ther. 2023. PMID: 36721147 Free PMC article. - Nutraceutical Approach to Preventing Coronavirus Disease 2019 and Related Complications.
Derosa G, Maffioli P, D'Angelo A, Di Pierro F. Derosa G, et al. Front Immunol. 2021 Jun 28;12:582556. doi: 10.3389/fimmu.2021.582556. eCollection 2021. Front Immunol. 2021. PMID: 34262553 Free PMC article. Review. - The Impact of Curcumin on Immune Response: An Immunomodulatory Strategy to Treat Sepsis.
Allegra A, Mirabile G, Ettari R, Pioggia G, Gangemi S. Allegra A, et al. Int J Mol Sci. 2022 Nov 25;23(23):14710. doi: 10.3390/ijms232314710. Int J Mol Sci. 2022. PMID: 36499036 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous