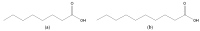Penetration Enhancers in Ocular Drug Delivery - PubMed (original) (raw)
Review
Penetration Enhancers in Ocular Drug Delivery
Roman V Moiseev et al. Pharmaceutics. 2019.
Abstract
There are more than 100 recognized disorders of the eye. This makes the development of advanced ocular formulations an important topic in pharmaceutical science. One of the ways to improve drug delivery to the eye is the use of penetration enhancers. These are defined as compounds capable of enhancing drug permeability across ocular membranes. This review paper provides an overview of anatomical and physiological features of the eye and discusses some common ophthalmological conditions and permeability of ocular membranes. The review also presents the analysis of literature on the use of penetration-enhancing compounds (cyclodextrins, chelating agents, crown ethers, bile acids and bile salts, cell-penetrating peptides, and other amphiphilic compounds) in ocular drug delivery, describing their properties and modes of action.
Keywords: cornea; ocular conditions; ocular drug delivery; ophthalmology; penetration enhancers.
Conflict of interest statement
The authors declare no conflicts of interest. Fraser Steele is the employee of the MC2 therapeutics. The company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures
Figure 1
Anatomy of the human eye: 1—cornea; 2—meibomian glands; 3—palpebral conjunctiva; 4—bulbar conjunctiva; 5—conjunctival fornix; 6—sclera; 7—iris; 8—anterior chamber; 9—iridocorneal angle; 10—ciliary body; 11—lens; 12—posterior chamber; 13—suspensory ligament; 14—choroid; 15—retinal pigmented epithelium; 16—retina; 17—vitreous body; 18—optic disc; 19—optic nerve; 20—central artery and vein of the retina; 21—fovea.
Figure 2
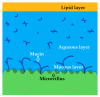
The tear film consists of the outer lipid layer, middle aqueous layer, and mucous layer.
Figure 3
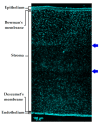
Micrograph demonstrating a cross-section of the multilayered structure of porcine cornea. Scale bar = 100 µm. Please note that this micrograph is a combination of three images stitched together using Inkscape 0.92.4 software due to the restrictions of the microscope camera field of view posing restrictions to the image in view. The arrows indicate stitches between images.
Figure 4
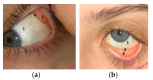
Three portions of conjunctiva: (a) 1—bulbar conjunctiva; 2—superior conjunctival fornix; 3—palpebral conjunctiva of the upper lid; (b) 1—bulbar conjunctiva; 2—inferior conjunctival fornix; 3—palpebral conjunctiva of the lower lid.
Figure 5
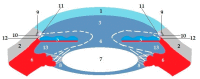
Pathways of aqueous humor outflow are indicated with arrows. 1—cornea; 2—sclera; 3—anterior chamber; 4—pupil; 5—iris; 6—ciliary body; 7—lens; 8—suspensory ligament; 9—Schlemm’s canal; 10—trabecular meshwork; 11—trabecular route; 12—uveoscleral route; 13—posterior chamber.
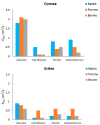
Figure 6
Effective diffusion coefficients Deff (cm2/s) of several drugs for rabbit, porcine, and bovine cornea and sclera. Data taken from Reference [21] with permission from Elsevier,2012.
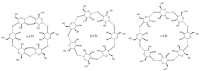
Figure 7
Structures of α-cyclodextrin, β-cyclodextrin, and γ-cyclodextrin. The image was reproduced under the Creative Commons Attribution Share Alike 3.0 Unported license [77].
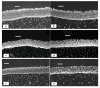
Figure 8
Micrographs of bovine cornea exposed to 1 mL of β-cyclodextrin (30 mg⋅mL−1) (b,d,f) against non-exposed regions (a,c,e). Exposure time: 15 (a,b), 45 (c,d), and 75 min (e,f). Scale bar = 100 µm. Reproduced from Reference [68] with permission from American Chemical Society, 2013.
Figure 9
Structures of ethylenediamine-N,N,_N_’,_N_’-tetraacetic acid (EDTA) (a), ethylene glycol-bis(beta-aminoethyl)-N,N,_N_’,_N_’-tetraacetic acid (EGTA) (b), 1,2-bis(o-aminophenoxy)ethane-N,N,_N_’,_N_’-tetraacetic acid (BAPTA) (c), and ethylenediamine-N,_N_’-disuccinic acid (EDDS) (d).
Figure 9
Structures of ethylenediamine-N,N,_N_’,_N_’-tetraacetic acid (EDTA) (a), ethylene glycol-bis(beta-aminoethyl)-N,N,_N_’,_N_’-tetraacetic acid (EGTA) (b), 1,2-bis(o-aminophenoxy)ethane-N,N,_N_’,_N_’-tetraacetic acid (BAPTA) (c), and ethylenediamine-N,_N_’-disuccinic acid (EDDS) (d).
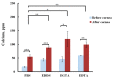
Figure 10
Calcium concentration in solutions containing phosphate-buffered saline (PBS), EDDS, EGTA, and EDTA (1 mg⋅mL−1) before and after 3 h of exposure to bovine cornea. * p < 0.05, ** p < 0.01, *** p < 0.001; one-way ANOVA; n = 3. Reproduced from Reference [17] under the terms of the Creative Commons Attribution License (CC BY).
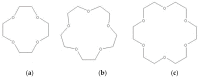
Figure 11
Structures of 12-crown-4 (a), 15-crown-5 (b), and 18-crown-6 (c).
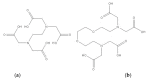
Figure 12
Structures of digitonin (a) and benzalkonium chloride (b).
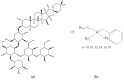
Figure 13
Structures of deoxycholate (a), glycocholate (b), and taurodeoxycholate (c).
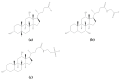
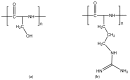
Figure 14
Structures of poly-
l
-serine (a) and poly-
l
-arginine hydrochloride (b).
Figure 15
Structures of caprylic acid (a) and capric acid (b).
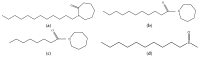
Figure 16
Structures of Azone™ (a), hexamethylenelauramide (b), hexamethyleneoctanamide (c), and decylmethylsulfoxide (d).
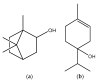
Figure 17
Structures of borneol (a) and terpinen-4-ol (b).
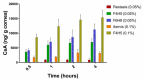
Figure 18
The cumulative amount of dorzolamide hydrochloride in the receiver chamber of a vertical Franz diffusion cell from ophthalmic gel formulations with various terpinen-4-ol concentrations and a fixed concentration of Carbopol-934 through the excised rabbit’s cornea (n = 3). Reproduced from Reference [141] with permission from Elsevier, 2010.
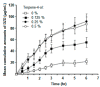
Figure 19
Corneal penetration of cyclosporin A (CsA) from the test formulations. The amount of CsA recovered (ng) per g of cornea after application of a single 50-µL dose was plotted against time (n = 5; mean ± standard error of the mean (SEM)). Reproduced from Reference [142] with permission from Elsevier, 2018.
Similar articles
- Crown Ethers: Novel Permeability Enhancers for Ocular Drug Delivery?
Morrison PWJ, Porfiryeva NN, Chahal S, Salakhov IA, Lacourt C, Semina II, Moustafine RI, Khutoryanskiy VV. Morrison PWJ, et al. Mol Pharm. 2017 Oct 2;14(10):3528-3538. doi: 10.1021/acs.molpharmaceut.7b00556. Epub 2017 Aug 30. Mol Pharm. 2017. PMID: 28825493 - Topical Ocular Drug Delivery: The Impact of Permeation Enhancers.
Santos G, Delgado E, Silva B, Braz BS, Gonçalves L. Santos G, et al. Pharmaceutics. 2025 Mar 31;17(4):447. doi: 10.3390/pharmaceutics17040447. Pharmaceutics. 2025. PMID: 40284442 Free PMC article. Review. - Enhancement of ocular drug penetration.
Sasaki H, Yamamura K, Mukai T, Nishida K, Nakamura J, Nakashima M, Ichikawa M. Sasaki H, et al. Crit Rev Ther Drug Carrier Syst. 1999;16(1):85-146. Crit Rev Ther Drug Carrier Syst. 1999. PMID: 10099899 Review. - Penetration enhancers and ocular bioadhesives: two new avenues for ophthalmic drug delivery.
Kaur IP, Smitha R. Kaur IP, et al. Drug Dev Ind Pharm. 2002 Apr;28(4):353-69. doi: 10.1081/ddc-120002997. Drug Dev Ind Pharm. 2002. PMID: 12056529 Review. - Understanding the influence of surface properties of nanoparticles and penetration enhancers for improving bioavailability in eye tissues in vivo.
Mahaling B, Katti DS. Mahaling B, et al. Int J Pharm. 2016 Mar 30;501(1-2):1-9. doi: 10.1016/j.ijpharm.2016.01.053. Epub 2016 Jan 25. Int J Pharm. 2016. PMID: 26821059
Cited by
- An Update on Strategies to Deliver Protein and Peptide Drugs to the Eye.
Boddu SHS, Acharya D, Hala V, Jani H, Pande S, Patel C, Shahwan M, Jwala R, Ranch KM. Boddu SHS, et al. ACS Omega. 2023 Sep 22;8(39):35470-35498. doi: 10.1021/acsomega.3c02897. eCollection 2023 Oct 3. ACS Omega. 2023. PMID: 37810716 Free PMC article. Review. - Multifunctional nano-in-micro delivery systems for targeted therapy in fundus neovascularization diseases.
Liu X, Huang K, Zhang F, Huang G, Wang L, Wu G, Ren H, Yang G, Lin Z. Liu X, et al. J Nanobiotechnology. 2024 Jun 20;22(1):354. doi: 10.1186/s12951-024-02614-1. J Nanobiotechnology. 2024. PMID: 38902775 Free PMC article. Review. - Peptidomimetics Therapeutics for Retinal Disease.
Parsons DE, Lee SH, Sun YJ, Velez G, Bassuk AG, Smith M, Mahajan VB. Parsons DE, et al. Biomolecules. 2021 Feb 24;11(3):339. doi: 10.3390/biom11030339. Biomolecules. 2021. PMID: 33668179 Free PMC article. Review. - Drug Delivery Challenges and Current Progress in Nanocarrier-Based Ocular Therapeutic System.
Akhter MH, Ahmad I, Alshahrani MY, Al-Harbi AI, Khalilullah H, Afzal O, Altamimi ASA, Najib Ullah SNM, Ojha A, Karim S. Akhter MH, et al. Gels. 2022 Jan 28;8(2):82. doi: 10.3390/gels8020082. Gels. 2022. PMID: 35200463 Free PMC article. Review. - Posterior Segment Ophthalmic Drug Delivery: Role of Muco-Adhesion with a Special Focus on Chitosan.
Burhan AM, Klahan B, Cummins W, Andrés-Guerrero V, Byrne ME, O'Reilly NJ, Chauhan A, Fitzhenry L, Hughes H. Burhan AM, et al. Pharmaceutics. 2021 Oct 14;13(10):1685. doi: 10.3390/pharmaceutics13101685. Pharmaceutics. 2021. PMID: 34683978 Free PMC article. Review.
References
- World Health Organization. [(accessed on 12 December 2018)]; Available online: https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-im....
- Morrison P.W.J., Khutoryanskiy V.V. Anatomy of the Eye and the Role of Ocular Mucosa in Drug Delivery. In: Khutoryanskiy V.V., editor. Mucoadhesive Materials and Drug Delivery Systems. 1st ed. John Wiley & Sons, Ltd.; Chichester, UK: 2014. pp. 39–60.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources