Diagnosis and management of individuals with Fetal Valproate Spectrum Disorder; a consensus statement from the European Reference Network for Congenital Malformations and Intellectual Disability - PubMed (original) (raw)
doi: 10.1186/s13023-019-1064-y.
Rebecca Bromley 3 4, John Dean 5, Hubert Journel 6, Sylvie Odent 7, Amanda Wood 8 9, Janet Williams 10, Verna Cuthbert 11, Latha Hackett 12, Neelo Aslam 12, Heli Malm 13, Gregory James 14 15 16 17, Lena Westbom 18, Ruth Day 19, Edmund Ladusans 20, Adam Jackson 21, Iain Bruce 22, Robert Walker 23, Sangeet Sidhu 24, Catrina Dyer 25, Jane Ashworth 26, Daniel Hindley 27, Gemma Arca Diaz 28, Myfanwy Rawson 29, Peter Turnpenny 30
Affiliations
- PMID: 31324220
- PMCID: PMC6642533
- DOI: 10.1186/s13023-019-1064-y
Diagnosis and management of individuals with Fetal Valproate Spectrum Disorder; a consensus statement from the European Reference Network for Congenital Malformations and Intellectual Disability
Jill Clayton-Smith et al. Orphanet J Rare Dis. 2019.
Abstract
Background: A pattern of major and minor congenital anomalies, facial dysmorphic features, and neurodevelopmental difficulties, including cognitive and social impairments has been reported in some children exposed to sodium valproate (VPA) during pregnancy. Recognition of the increased risks of in utero exposure to VPA for congenital malformations, and for the neurodevelopmental effects in particular, has taken many years but these are now acknowledged following the publication of the outcomes of several prospective studies and registries. As with other teratogens, exposure to VPA can have variable effects, ranging from a characteristic pattern of major malformations and significant intellectual disability to the other end of the continuum, characterised by facial dysmorphism which is often difficult to discern and a more moderate effect on neurodevelopment and general health. It has become clear that some individuals with FVSD have complex needs requiring multidisciplinary care but information regarding management is currently lacking in the medical literature.
Methods: An expert group was convened by ERN-ITHACA, the European Reference Network for Congenital Malformations and Intellectual Disability comprised of professionals involved in the care of individuals with FVSD and with patient representation. Review of published and unpublished literature concerning management of FVSD was undertaken and the level of evidence from these sources graded. Management recommendations were made based on strength of evidence and consensus expert opinion, in the setting of an expert consensus meeting. These were then refined using an iterative process and wider consultation.
Results: Whilst there was strong evidence regarding the increase in risk for major congenital malformations and neurodevelopmental difficulties there was a lack of high level evidence in other areas and in particular in terms of optimal clinical management.. The expert consensus approach facilitated the formulation of management recommendations, based on literature evidence and best practice. The outcome of the review and group discussions leads us to propose the term Fetal Valproate Spectrum Disorder (FVSD) as we feel this better encompasses the broad range of effects seen following VPA exposure in utero.
Conclusion: The expert consensus approach can be used to define the best available clinical guidance for the diagnosis and management of rare disorders such as FVSD. FVSD can have medical, developmental and neuropsychological impacts with life-long consequences and affected individuals benefit from the input of a number of different health professionals.
Keywords: Antiepileptic drug; Expert consensus; Fetal valproate syndrome; Guideline; Management; Teratogen.
Conflict of interest statement
All participants in the process were asked to declare any conflicts of interest or competing interests. The authors declared that they had no competing interests.
Figures
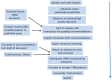
Fig. 1
Outline Of The Process Of Producing The Consensus Statement
Fig. 2
Facial Features Associated With Valproate Exposure At Different Ages. Note the presence of anteverted nares, small mouth, thin upper lip, everted lower lip, flattening of philtrum, prominent midline to forehead. Features are attenuated but still apparent in young adult
Fig. 3
Minor limb malformations Seen After VPA exposure. Note the hypoplastic and overlapping toes and flattening of the arches due to the joint laxity frequently seen in FVS
Similar articles
- Neurodevelopmental outcomes in children and adults with Fetal Valproate Spectrum Disorder: A contribution from the ConcePTION project.
Bluett-Duncan M, Astill D, Charbak R, Clayton-Smith J, Cole S, Cook PA, Cozens J, Keely K, Morris J, Mukherjee R, Murphy E, Turnpenny P, Williams J, Wood AG, Yates LM, Bromley RL. Bluett-Duncan M, et al. Neurotoxicol Teratol. 2023 Nov-Dec;100:107292. doi: 10.1016/j.ntt.2023.107292. Epub 2023 Sep 4. Neurotoxicol Teratol. 2023. PMID: 37666366 - Valproate prescribing practices for women with intellectual disability across Europe.
Watkins L, Reuber M, Perera B, Courtenay K, Banks R, Murphy E, Angus-Leppan H, Shankar R. Watkins L, et al. Acta Neurol Scand. 2021 Jan;143(1):56-61. doi: 10.1111/ane.13337. Epub 2020 Sep 6. Acta Neurol Scand. 2021. PMID: 32813274 - Modified Xenopus laevis approach (R-FETAX) as an alternative test for the evaluation of foetal valproate spectrum disorder.
Battistoni M, Bacchetta R, Di Renzo F, Metruccio F, Moretto A, Menegola E. Battistoni M, et al. Reprod Toxicol. 2022 Jan;107:140-149. doi: 10.1016/j.reprotox.2021.12.005. Epub 2021 Dec 16. Reprod Toxicol. 2022. PMID: 34923091 - Chromatin Imbalance as the Vertex Between Fetal Valproate Syndrome and Chromatinopathies.
Parodi C, Di Fede E, Peron A, Viganò I, Grazioli P, Castiglioni S, Finnell RH, Gervasini C, Vignoli A, Massa V. Parodi C, et al. Front Cell Dev Biol. 2021 Apr 20;9:654467. doi: 10.3389/fcell.2021.654467. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33959609 Free PMC article. Review. - Treatment for epilepsy in pregnancy: neurodevelopmental outcomes in the child.
Bromley R, Weston J, Adab N, Greenhalgh J, Sanniti A, McKay AJ, Tudur Smith C, Marson AG. Bromley R, et al. Cochrane Database Syst Rev. 2014 Oct 30;2014(10):CD010236. doi: 10.1002/14651858.CD010236.pub2. Cochrane Database Syst Rev. 2014. PMID: 25354543 Free PMC article. Review.
Cited by
- The first review on prenatal drug exposure and ocular malformation occurrence.
Dubucs C, Plaisancié J, Courtade-Saidi M, Damase-Michel C. Dubucs C, et al. Front Pediatr. 2024 Sep 4;12:1379875. doi: 10.3389/fped.2024.1379875. eCollection 2024. Front Pediatr. 2024. PMID: 39296666 Free PMC article. Review. - Valproate Targets Mammalian Gastrulation Impairing Neural Tissue Differentiation and Development of the Placental Source In Vitro.
Katušić-Bojanac A, Plazibat M, Himelreich-Perić M, Eck-Raković K, Krasić J, Sinčić N, Jurić-Lekić G, Ježek D, Bulić-Jakuš F. Katušić-Bojanac A, et al. Int J Mol Sci. 2022 Aug 9;23(16):8861. doi: 10.3390/ijms23168861. Int J Mol Sci. 2022. PMID: 36012122 Free PMC article. - Gastrointestinal dysfunction in the valproic acid induced model of social deficit in rats.
Varley AN, Browning KN. Varley AN, et al. Auton Neurosci. 2024 Jun;253:103161. doi: 10.1016/j.autneu.2024.103161. Epub 2024 Feb 29. Auton Neurosci. 2024. PMID: 38461695 - A transient time window for early predispositions in newborn chicks.
Versace E, Ragusa M, Vallortigara G. Versace E, et al. Sci Rep. 2019 Dec 10;9(1):18767. doi: 10.1038/s41598-019-55255-y. Sci Rep. 2019. PMID: 31822755 Free PMC article. - Hdac4 Regulates the Proliferation of Neural Crest-Derived Osteoblasts During Murine Craniofacial Development.
Ha N, Sun J, Bian Q, Wu D, Wang X. Ha N, et al. Front Physiol. 2022 Feb 15;13:819619. doi: 10.3389/fphys.2022.819619. eCollection 2022. Front Physiol. 2022. PMID: 35242053 Free PMC article.
References
- New measures to avoid valproate exposure in pregnancy endorsed. European Medicines Agency. 2018: EMA/145600/2018 Available from. https://www.ema.europa.eu/documents/referral/valproate-article-31-referr.... Accessed 31 May 2018.
- Marson AG, Al-Kharusi AM, Alwaidh M, Appleton R, Baker GA, Chadwick DW, et al. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalised and unclassifiable epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369(9566):1016–1026. doi: 10.1016/S0140-6736(07)60461-9. - DOI - PMC - PubMed
- Robert E, Guibaud P. Maternal valproic acid and congenital neural tube defects. Lancet. 1982;2:1282–1283. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials