Methodological steps used by authors of systematic reviews and meta-analyses of clinical trials: a cross-sectional study - PubMed (original) (raw)
doi: 10.1186/s12874-019-0780-2.
Ali Mahmoud Ahmed 2 3, Reem Yousry Fala 2 4, Mohamed Magdy Khattab 2 3, Mona Hassan Ahmed Othman 2 5, Sara Attia Mahmoud Abdelrahman 2 6, Le Phuong Thao 2 7, Ahmed Elsaid Abd Elsamie Gabl 2 8, Samar Ahmed Elrashedy 2 9, Peter N Lee 10, Kenji Hirayama 11, Hosni Salem 12, Nguyen Tien Huy 13 14
Affiliations
- PMID: 31349805
- PMCID: PMC6659247
- DOI: 10.1186/s12874-019-0780-2
Methodological steps used by authors of systematic reviews and meta-analyses of clinical trials: a cross-sectional study
Hoang Thi Nam Giang et al. BMC Med Res Methodol. 2019.
Abstract
Background: The quality of systematic reviews and meta-analyses (SR/MAs) depends on the extent of the methods used. We investigated the methodological steps used by authors of SR/MAs of clinical trials via an author survey.
Methods: We conducted an email-based cross-sectional study by contacting corresponding authors of SR/MAs that were published in 2015 and 2016 and retrieved through the PubMed database. The 27-item questionnaire was developed to study the methodological steps used by authors when conducting a SR/MA and the demographic characteristics of the respondent. Besides the demographic characteristics, methodological questions regarding the source, extraction and synthesis of data were included.
Results: From 10,292 emails sent, 384 authors responded and were included in the final analysis. Manual searches were carried out by 69.2% of authors, while 87.3% do updated searches, 49.2% search grey literature, 74.9% use the Cochrane tool for risk of bias assessment, 69.8% assign more than two reviewers for data extraction, 20.5% use digital software to extract data from graphs, 57.9% use raw data in the meta-analysis, and 43.8% meta-analyze both adjusted and non-adjusted data. There was a positive correlation of years of experience in conducting of SR/MAs with both searching grey literature (P = 0.0003) and use of adjusted and non-adjusted data (P = 0.006).
Conclusions: Many authors still do not carry out many of the vital methodological steps to be taken when performing any SR/MA. The experience of the authors in SR/MAs is highly correlated with use of the recommended tips for SR/MA conduct. The optimal methodological approach for researchers conducting a SR/MA should be standardized.
Keywords: Cross sectional study; Data extraction; Meta-analysis; Systematic review.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
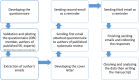
Fig. 1
The flow chart of our study explaining the steps of data collection, handling, and reporting
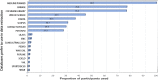
Fig. 2
The proportions of respondents searching each database
Similar articles
- Protocol registration issues of systematic review and meta-analysis studies: a survey of global researchers.
Tawfik GM, Giang HTN, Ghozy S, Altibi AM, Kandil H, Le HH, Eid PS, Radwan I, Makram OM, Hien TTT, Sherif M, Hossain AS, Thang TLL, Puljak L, Salem H, Numair T, Moji K, Huy NT. Tawfik GM, et al. BMC Med Res Methodol. 2020 Aug 25;20(1):213. doi: 10.1186/s12874-020-01094-9. BMC Med Res Methodol. 2020. PMID: 32842968 Free PMC article. - Abstract analysis method facilitates filtering low-methodological quality and high-bias risk systematic reviews on psoriasis interventions.
Gómez-García F, Ruano J, Aguilar-Luque M, Alcalde-Mellado P, Gay-Mimbrera J, Hernández-Romero JL, Sanz-Cabanillas JL, Maestre-López B, González-Padilla M, Carmona-Fernández PJ, García-Nieto AV, Isla-Tejera B. Gómez-García F, et al. BMC Med Res Methodol. 2017 Dec 29;17(1):180. doi: 10.1186/s12874-017-0460-z. BMC Med Res Methodol. 2017. PMID: 29284417 Free PMC article. - Contacting of authors by systematic reviewers: protocol for a cross-sectional study and a survey.
Meursinge Reynders R, Ladu L, Di Girolamo N. Meursinge Reynders R, et al. Syst Rev. 2017 Dec 8;6(1):249. doi: 10.1186/s13643-017-0643-z. Syst Rev. 2017. PMID: 29216930 Free PMC article. - The quality of systematic reviews/meta-analyses published in the field of bariatrics: A cross-sectional systematic survey using AMSTAR 2 and ROBIS.
Storman M, Storman D, Jasinska KW, Swierz MJ, Bala MM. Storman M, et al. Obes Rev. 2020 May;21(5):e12994. doi: 10.1111/obr.12994. Epub 2020 Jan 29. Obes Rev. 2020. PMID: 31997545 Review. - Assessment of the abstract reporting of systematic reviews of dose-response meta-analysis: a literature survey.
Jia PL, Xu B, Cheng JM, Huang XH, Kwong JSW, Liu Y, Zhang C, Han Y, Xu C. Jia PL, et al. BMC Med Res Methodol. 2019 Jul 15;19(1):148. doi: 10.1186/s12874-019-0798-5. BMC Med Res Methodol. 2019. PMID: 31307388 Free PMC article. Review.
Cited by
- Efficacy of ultrasound-guided stellate ganglion block in relieving acute postoperative pain: a systematic review and meta-analysis.
Zhao Y, Xiao X. Zhao Y, et al. J Int Med Res. 2024 May;52(5):3000605241252237. doi: 10.1177/03000605241252237. J Int Med Res. 2024. PMID: 38759220 Free PMC article. - The Effects of Responsible Gambling Pop-Up Messages on Gambling Behaviors and Cognitions: A Systematic Review and Meta-Analysis.
Bjørseth B, Simensen JO, Bjørnethun A, Griffiths MD, Erevik EK, Leino T, Pallesen S. Bjørseth B, et al. Front Psychiatry. 2021 Jan 25;11:601800. doi: 10.3389/fpsyt.2020.601800. eCollection 2020. Front Psychiatry. 2021. PMID: 33569015 Free PMC article. - Problematic Gaming and Sleep: A Systematic Review and Meta-Analysis.
Kristensen JH, Pallesen S, King DL, Hysing M, Erevik EK. Kristensen JH, et al. Front Psychiatry. 2021 Jun 7;12:675237. doi: 10.3389/fpsyt.2021.675237. eCollection 2021. Front Psychiatry. 2021. PMID: 34163386 Free PMC article. - Guideline on allergen immunotherapy in IgE-mediated allergic diseases: S2K Guideline of the German Society of Allergology and Clinical Immunology (DGAKI), Society of Pediatric Allergology and Environmental Medicine (GPA), Medical Association of German Allergologists (AeDA), Austrian Society of Allergology and Immunology (ÖGAI), Swiss Society for Allergology and Immunology (SSAI), German Dermatological Society (DDG), German Society of Oto-Rhino-Laryngology, Head and Neck Surgery (DGHNO-KHC), German Society of Pediatrics and Adolescent Medicine (DGKJ), Society of Pediatric Pulmonology (GPP), German Respiratory Society (DGP), German Professional Association of Otolaryngologists (BVHNO), German Association of Paediatric and Adolescent Care Specialists (BVKJ), Federal Association of Pneumologists, Sleep and Respiratory Physicians (BdP), Professional Association of German Dermatologists (BVDD).
Pfaar O, Ankermann T, Augustin M, Bubel P, Böing S, Brehler R, Eng PA, Fischer PJ, Gerstlauer M, Hamelmann E, Jakob T, Kleine-Tebbe J, Kopp MV, Lau S, Mülleneisen N, Müller C, Nemat K, Pfützner W, Saloga J, Strömer K, Schmid-Grendelmeier P, Schuster A, Sturm GJ, Taube C, Szépfalusi Z, Vogelberg C, Wagenmann M, Wehrmann W, Werfel T, Wöhrl S, Worm M, Wedi B; Commenting participation and process support:; Kaul S, Mahler V, Schwalfenberg A. Pfaar O, et al. Allergol Select. 2022 Sep 6;6:167-232. doi: 10.5414/ALX02331E. eCollection 2022. Allergol Select. 2022. PMID: 36178453 Free PMC article.
References
- Higgins JPT, Green S (editors). Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated march 2011]. Cochrane Collaboration, 2011. Available from http://handbook.cochrane.org.
- Hung Bui The, Long Nguyen Phuoc, Hung Le Phi, Luan Nguyen Thien, Anh Nguyen Hoang, Nghi Tran Diem, Van Hieu Mai, Trang Nguyen Thi Huyen, Rafidinarivo Herizo Fabien, Anh Nguyen Ky, Hawkes David, Huy Nguyen Tien, Hirayama Kenji. Research Trends in Evidence-Based Medicine: A Joinpoint Regression Analysis of More than 50 Years of Publication Data. PLOS ONE. 2015;10(4):e0121054. doi: 10.1371/journal.pone.0121054. - DOI - PMC - PubMed
- Esterhuizen TM, Thabane L. Con: Meta-analysis: some key limitations and potential solutions. Nephrol Dial Transplant. 2016;31(6):882–5. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials