Peptide Tectonics: Encoded Structural Complementarity Dictates Programmable Self-Assembly - PubMed (original) (raw)
Review
. 2019 Apr 29;6(13):1802043.
doi: 10.1002/advs.201802043. eCollection 2019 Jul 3.
Affiliations
- PMID: 31380179
- PMCID: PMC6662064
- DOI: 10.1002/advs.201802043
Review
Peptide Tectonics: Encoded Structural Complementarity Dictates Programmable Self-Assembly
Shaofeng Lou et al. Adv Sci (Weinh). 2019.
Abstract
Programmable self-assembly of peptides into well-defined nanostructures represents one promising approach for bioinspired and biomimetic synthesis of artificial complex systems and functional materials. Despite the progress made over the past two decades in the development of strategies for precise manipulation of the self-assembly of peptides, there is a remarkable gap between current peptide assemblies and biological systems in terms of structural complexity and functions. Here, the concept of peptide tectonics for the creation of well-defined nanostructures predominately driven by the complementary association at the interacting interfaces of tectons is introduced. Peptide tectons are defined as peptide building blocks exhibiting structural complementarity at the interacting interfaces of commensurate domains and undergoing programmable self-assembly into defined supramolecular structures promoted by complementary interactions. Peptide tectons are categorized based on their conformational entropy and the underlying mechanism for the programmable self-assembly of peptide tectons is highlighted focusing on the approaches for incorporating the structural complementarity within tectons. Peptide tectonics not only provides an alternative perspective to understand the self-assembly of peptides, but also allows for precise manipulation of peptide interactions, thus leading to artificial systems with advanced complexity and functions and paves the way toward peptide-related functional materials resembling natural systems.
Keywords: biomaterials; conformational entropy; hierarchical nanostructures; peptides; self‐assembly; supramolecular chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
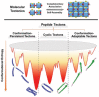
Scheme 1
The pathway in biological systems for synthesis of sophisticated proteins and the strategy of peptide tectonics to create comparable bioinspired or biomimetic systems via rational control over the interfacial association between structural complementarity at predictable interfaces.
Scheme 2
Schematic representation of (top) molecular tectonics to create 2D networks driven by complementary noncovalent interactions and (bottom) the classification of peptide tectons into three categories based on the conformational stability of incorporated domains and their conformational space, which simultaneously govern the conformational entropy of tectons. The persistence of the conformation of peptide tectons refers to the secondary structures retained in the monomeric and assembled states.
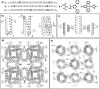
Figure 1
a) SAF coiled coils consisting of two linear peptide tectons with complementary charges. b) The electrostatic interactions, in combination with the H‐bonds involving the amide group of Asn residues, led to formation of staggered, parallel heterodimers with “sticky ends.” c) Nanofibers formed by SAF coiled coils. The yellow rectangles in (b) denote the Asn pairs. Reproduced with permission.14 Copyright 2000, American Chemical Society.
Figure 2
Design of a) T‐shaped, c) FiSh, and e) MaP peptide tectons starting from the SAF peptides with complementary charges. b,d,f) Using four SAF domains, i.e., AN, BC, CN, and DC (the superscripts denote the free terminus of the domains in entire peptides), T‐shaped, FiSh, and MaP peptide tectons were designed via rational combination and coassembled with the SAF peptides into branched or networked coiled‐coil nanostructures. Yellow and red signals denote the Asn residues within the BC and CN half domains, respectively. a,b) Reproduced with permission.18 Copyright 2003, Wiley‐VCH. c,d) Reproduced with permission.19 Copyright 2003, Nature Publishing Group. e,f) Reproduced with permission.20 Copyright 2005, American Chemical Society.
Figure 3
Design of virus‐like architectures. Top: a) Sequence of the trifaceted coiled‐coil tecton and its coiled‐coil subunit under helical wheel pattern with 3.5 residues per turn. The cysteine bridge is highlighted in yellow. The clockwise and anticlockwise arrows indicate intra‐ (c_–_g) and interhelical (_c_–_e_′) electrostatic interactions, respectively. b) Formation of virus‐like shells by assembling the trifaceted helix tectons at three facets and cross‐linking cysteine residues. c) 3D ribbon models of virus‐like shells assembled without (left) and with siRNA (right). Reproduced with permission.22 Copyright 2016, American Chemical Society. Bottom: d) Sequence of the positively charged C1(+) chain in the dendrimer C3‐hub and the negatively charged C1(−) chain aligned under the coiled‐coil heptad repeat pattern (top) and the helical wheel pattern (bottom). The cysteine unit is highlighted in yellow. e) The chemical structure of the dendrimer C3‐hub (C3(+) strand). βA refers to β‐alanine. f) Molecular model of the C3‐triskelion subunit. Each subunit corresponds to the altitude of an equilateral triangle. g) T = 4 icosahedron with four facets in each triangular face occupied by one triskelion. h) Model of a T = 4 capsid assembled from the C3‐triskelion, in which one of the fivefold (left) or sixfold (right) axes is highlighted. Reproduced under the terms of the Creative Commons 4.0 license.23 Copyright 2017, Nature Publishing Group.
Figure 4
Coiled‐coil peptides GCN4‐p2L functionalized with NTA and His2 ligands at termini. a) Helical wheel representation and sequence of the GCN4‐p2L peptide. b) Schematic illustration of the metal coordination–triggered head‐to‐tail assembly of the GCN4‐p2L trimeric coiled coil. c) Scanning electron microscopy (SEM) images of the nanostructures formed by peptide GCN4‐p2L and divalent metal ions in a molar ratio of 1:0.4. d) Graphical representation of the spatial capture of cargoes within the nanostructures. Reproduced with permission.26 Copyright 2016, American Chemical Society.
Figure 5
a) Sequences of peptides 1, 2, and 3, chemical structure of the non‐natural residue X containing Tpy unit, and the coordinating model of the Tpy ligand with Cu2+ involving a Glu residue. b) Schematic representation of the dimeric, trimeric, and tetrameric coiled coils formed by peptides 1, 2, and 3, respectively. Crystal structures: c) 1D coordination polymer formed by laterally organized peptide 1, d) extended framework formed by peptide 2, and e) 2D net formed by peptide 3 based on the Tpy–Cu2+–Glu linkages. Reproduced with permission.27 Copyright 2017, American Chemical Society.
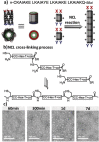
Figure 6
a) Peptide sequence of the coiled coils terminated with thiol and thioester groups and schematic representation of covalently linking αHBs leading to formation of long nanotubes. b) The oligomerization of αHBs induced by NCL reactions. c) Transmission electron microscopy (TEM) images of resulting nanotubes stabilized by the NCL reaction, as evident from increase of the length of nanofibers at different times. Reproduced under the terms of the Creative Commons license.28 Copyright 2015, Wiley‐VCH.
Figure 7
Linear CMP tectons consisting of the domains with complementary charges. Chemical structures for the CMPs containing a) PRG and EOG (R–O–E model) or b) PKG and DOG domains (K–O–D model) linked by a neutral POG domain, as well as c) PKG and DOG domains linked by two neutral POG domains (O–K–D model). The representative stacking fashion of individual CMPs stabilized by the salt‐bridge interactions, and the corresponding morphology of the resulting nanostructures investigated by TEM are shown. a) Reproduced with permission.33 Copyright 2007, American Chemical Society. b) Reproduced with permission.34 Copyright 2011, Nature Publishing Group. c) Reproduced with permission.35 Copyright 2011, Nature Publishing Group.
Figure 8
a) Self‐assembly of CMPs functionalized with NTA and His2 ligands at termini (NCoH) promoted by metal coordination into microflorettes formed by NCoH and Zn2+ ion in a molar ratio of 1:0.4. Reproduced with permission.40 Copyright 2009, American Chemical Society. b) Self‐assembly of a pair of symmetric CMPs functionalized with Ida (IdaCol) and His2 (HisCol) ligands induced by metal coordination into petal‐like nanostructures in the presence of 2 equiv. of Zn2+. Reproduced with permission.42 Copyright 2011, American Chemical Society.
Figure 9
a) Chemical structures of collagen‐mimetic peptides containing one, two, or three H‐Byp at the internal tripeptide repeats, leading to CMP H‐byp, H‐(byp)2, or H‐(byp)3, respectively. Upon addition of Fe3+ ion (1 equiv. to peptides), the CMPs assembled into b) branched fibers, c) round disk‐like structures, and d) hollow spheres based on the metal coordination, in addition to the π–π stacking interactions among aromatic ligands. b) Reproduced with permission.43 Copyright 2008, American Chemical Society. c) Reproduced with permission.43 Copyright 2010, American Chemical Society. d) Reproduced with permission.43 Copyright 2013, American Chemical Society.
Figure 10
Schematic representation of the proposed mechanism of formation of twisted or flat nanostructures based on the electrostatic repulsion or attraction among peptides EF4E, EF4K, EF4E/KF4K equimolar mixture, and KF4K. Reproduced with permission.3 Copyright 2016, American Chemical Society.
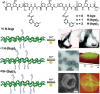
Figure 11
Linear asymmetric β‐sheet peptide tectons and their self‐assembly based on selective association among peptide domains. Top: Schematic representation of the self‐assembly of dual‐domain peptides into a) nanoribbons or b) nanofibers, respectively, dependent on the relative position of the alkyl tails within the isomeric linkages and the peptide domains. Reproduced with permission.49 Copyright 2016, American Chemical Society. Bottom: c) Self‐assembly of the linear peptides containing a hexaphenylalanine domain and an undecyl tail into Janus nanosheets, which was characterized by TEM and atomic force microscopy (AFM). The Janus nanosheets can be functionalized by biotin groups at one face to selectively capture avidin enzymes for catalyzing oxidation reactions. Reproduced under the terms of the Creative Commons license.51 Copyright 2017, American Chemical Society.
Figure 12
a) Self‐assembly of a β‐annulus peptide tailored from TBSV capsid into virus‐like nanocapsules. Reproduced with permission.52 Copyright 2010, Wiley‐VCH. b) Self‐assembly of a triskel capsid conjugate into virus‐like capsules with antimicrobial activity. Reproduced under the terms of the Creative Commons 3.0 license.53 Copyright 2016, Royal Society of Chemistry.
Figure 13
Peptide tectons derived from β‐hairpin peptides consisting of alternating valine and lysine residues flanking a –VPPT– loop. Top: a) Effect of the turn loop on peptide conformation and the morphology of resulting nanostructures, b,d) where –DPro–LPro– in the central turn allows the peptides to adopt a β‐hairpin conformation and self‐assemble into nanofibers, c,e) whereas peptides with –LPro–LPro– in the loop adopt an extended conformation and assemble into nanoribbons. d,e) TEM images of the resulting nanofibers and nanoribbons. b,d) Reproduced with permission.56 Copyright 2002, American Chemical Society. c,e): Reproduced with permission.59 Copyright 2005, American Chemical Society. Bottom: β‐Hairpins containing an exchangeable domain appended to β‐hairpin motifs. f) Sequence of the β‐hairpin and schematic representation of g) their dimerization via swapping between the exchangeable strands and h) formed hydrogels composed of nanofibrils. Reproduced with permission.60 Copyright 2008, American Chemical Society.
Figure 14
a) Helical wheel representation of peptide TZ1H trimeric helices coordinating with silver and b) the sequence of peptide TZ1H. c) Schematic illustration of the self‐assembly of peptide TZ1H above the p_K_ a of histidine residues triggered by metal coordination: a conformational transition from random coil to helix triggered by metal coordination led to formation of trimeric coiled coils that self‐assembled into nanofibers. d) TEM image of resulting nanofibers formed by peptide TZ1H and Ag+ in an equimolar ratio. Reproduced with permission.63 Copyright 2008, American Chemical Society.
Figure 15
Self‐assembly of bifaceted cyclopeptides consisting of two domains with a 1 + 1 + 1 or 2 + 1 pattern for the heptads. Top: a) Schematic representation of cyclopeptide 1 containing the heptads connected in 1 + 1 + 1 symmetric model by triglycyl linkers in an antiparallel direction and the arbitrary assembly of the tectons promoted by parallel coiled‐coil heterodimers formed by the charge‐complementary domains from neighboring cyclopeptides. b) SEM, differential interference contrast, and AFM images of the nanostructures formed by cyclopeptide 1. Reproduced with permission.65 Copyright 2012, Wiley‐VCH. Bottom: c) Schematic representation of cyclopeptide 2 containing the heptads connected in 2 + 1 asymmetric model within the two domains and simplified representation of the tecton assembly with one‐, two‐, and three‐heptad complementary overlaps. d) AFM, confocal, and optical microscopy images of the networks formed by cyclopeptide 2. Blue and red boxes represent cationic and anionic heptads, respectively, while arrows denote electrostatic interactions between lysine and glutamate residues. Reproduced with permission.65 Copyright 2014, American Chemical Society.
Figure 16
Cyclic peptide structures with alternating
d
‐ and
l
‐amino acids adopting flat ring‐shaped conformation. a) Cyclic peptides assembled into nanotubes composed of ordered parallel arrays of individual monomers predominantly based on H‐bonds. Reproduced with permission.69 Copyright 1996, American Chemical Society. b) Cyclic peptides served as the scaffolds for phase‐separated polymers, leading to peptide–polymer conjugates with two corona configurations, which assembled into Janus nanotubes with “demixed” corona. Reproduced with permission.73 Copyright 2013, Nature Publishing Group.
Figure 17
Formation of nanotubes based on hierarchical self‐assembly of lanreotide peptides. Top: Schematic representation of the mechanism of self‐assembly of lanreotide peptides. a) Chemical structures of lanreotide adopting a β‐hairpin planar conformation stabilized by the disulfide bridge, the turn, and the intramolecular H‐bonds. b) Space‐filling models of lanreotide peptides with hydrophobic and hydrophilic faces. c) In the resulting nanotubes, two different β‐sheet strands superimposed with their C2 twofold axes, leading to the segregation of the aromatic and aliphatic moieties on each strand of β‐hairpins. The segregation of aromatic residues (red) from aliphatic residues (blue) and from hydrophilic region (green) is highlighted. Reproduced with permission.75 Copyright 2003, National Academy of Sciences. Bottom: Two strategies for modulating the diameter of the nanotubes formed by lanreotide peptides. d) The stacking models of lanreotide peptides within the two layers of nanotubes. Based on the stacking models, the diameter of nanotubes can be tuned based on either e) changing the geometrical size of the aromatic unit at the position for
d
‐Trp residue (Reproduced with permission.77 Copyright 2011, National Academy of Sciences) or f) incorporation of counterions for the free amine groups with lanreotide backbones (Reproduced with permission.77 Copyright 2012, American Chemical Society).
Figure 18
a) Macrocyclic peptides consisting of α‐helical and β‐sheet domains connected by hydrophilic flexible linkers. b) Due to the selective association among β‐sheet domains, the macrocyclic peptides assembled into spherical objects surrounded by α‐helices. c) TEM image of resulting spherical nanostructures. Reproduced with permission.80 Copyright 2009, Wiley‐VCH.
Similar articles
- Noncanonical Amino Acids Dictate Peptide Assembly in Living Cells.
Liu X, Hu B, Yu Z. Liu X, et al. Acc Chem Res. 2025 Apr 1;58(7):1081-1093. doi: 10.1021/acs.accounts.4c00796. Epub 2025 Mar 19. Acc Chem Res. 2025. PMID: 40105513 Review. - Nucleobase-Interaction-Directed Biomimetic Supramolecular Self-Assembly.
Sikder A, Esen C, O'Reilly RK. Sikder A, et al. Acc Chem Res. 2022 Jun 21;55(12):1609-1619. doi: 10.1021/acs.accounts.2c00135. Epub 2022 Jun 7. Acc Chem Res. 2022. PMID: 35671460 Free PMC article. - Squaring the circle in peptide assembly: from fibers to discrete nanostructures by de novo design.
Boyle AL, Bromley EH, Bartlett GJ, Sessions RB, Sharp TH, Williams CL, Curmi PM, Forde NR, Linke H, Woolfson DN. Boyle AL, et al. J Am Chem Soc. 2012 Sep 19;134(37):15457-67. doi: 10.1021/ja3053943. Epub 2012 Sep 6. J Am Chem Soc. 2012. PMID: 22917063 - Bioinspired Amino Acid Based Materials in Bionanotechnology: From Minimalistic Building Blocks and Assembly Mechanism to Applications.
Wang Y, Rencus-Lazar S, Zhou H, Yin Y, Jiang X, Cai K, Gazit E, Ji W. Wang Y, et al. ACS Nano. 2024 Jan 16;18(2):1257-1288. doi: 10.1021/acsnano.3c08183. Epub 2023 Dec 29. ACS Nano. 2024. PMID: 38157317 Review. - Reciprocal Self-Assembly of Peptide-DNA Conjugates into a Programmable Sub-10-nm Supramolecular Deoxyribonucleoprotein.
Kye M, Lim YB. Kye M, et al. Angew Chem Int Ed Engl. 2016 Sep 19;55(39):12003-7. doi: 10.1002/anie.201605696. Epub 2016 Aug 24. Angew Chem Int Ed Engl. 2016. PMID: 27553897
Cited by
- Design of Peptides that Fold and Self-Assemble on Graphite.
Legleiter J, Thakkar R, Velásquez-Silva A, Miranda-Carvajal I, Whitaker S, Tomich J, Comer J. Legleiter J, et al. J Chem Inf Model. 2022 Sep 12;62(17):4066-4082. doi: 10.1021/acs.jcim.2c00419. Epub 2022 Jul 26. J Chem Inf Model. 2022. PMID: 35881533 Free PMC article. - Supramolecular Architectures of Nucleic Acid/Peptide Hybrids.
Higashi SL, Rozi N, Hanifah SA, Ikeda M. Higashi SL, et al. Int J Mol Sci. 2020 Dec 12;21(24):9458. doi: 10.3390/ijms21249458. Int J Mol Sci. 2020. PMID: 33322664 Free PMC article. Review. - Fine structural tuning of the assembly of ECM peptide conjugates via slight sequence modifications.
Qin J, Sloppy JD, Kiick KL. Qin J, et al. Sci Adv. 2020 Oct 7;6(41):eabd3033. doi: 10.1126/sciadv.abd3033. Print 2020 Oct. Sci Adv. 2020. PMID: 33028534 Free PMC article. - Biomimetic peptide self-assembly for functional materials.
Levin A, Hakala TA, Schnaider L, Bernardes GJL, Gazit E, Knowles TPJ. Levin A, et al. Nat Rev Chem. 2020 Sep 15;4(11):615-634. doi: 10.1038/s41570-020-0215-y. Nat Rev Chem. 2020. PMID: 39650726 Free PMC article. - Controllable and Diversiform Topological Morphologies of Self-Assembling Supra-Amphiphiles with Aggregation-Induced Emission Characteristics for Mimicking Light-Harvesting Antenna.
Fu S, Su X, Li M, Song S, Wang L, Wang D, Tang BZ. Fu S, et al. Adv Sci (Weinh). 2020 Sep 23;7(20):2001909. doi: 10.1002/advs.202001909. eCollection 2020 Oct. Adv Sci (Weinh). 2020. PMID: 33101876 Free PMC article.
References
- a) Aida T., Meijer E. W., Stupp S. I., Science 2012, 335, 813; - PMC - PubMed
- b) Zhang S. G., Nat. Biotechnol. 2003, 21, 1171; - PubMed
- c) Hamley I. W., Angew. Chem., Int. Ed. 2007, 46, 8128; - PubMed
- d) Jung J. P., Gasiorowski J. Z., Collier J. H., Biopolymers 2010, 94, 49; - PMC - PubMed
- e) Yan X., Zhu P., Li J., Chem. Soc. Rev. 2010, 39, 1877; - PubMed
- f) Woolfson D. N., Mahmoud Z. N., Chem. Soc. Rev. 2010, 39, 3464; - PubMed
- g) Hendricks M. P., Sato K., Palmer L. C., Stupp S. I., Acc. Chem. Res. 2017, 50, 2440; - PMC - PubMed
- h) Baker E. G., Bartlett G. J., Porter Goff K. L., Woolfson D. N., Acc. Chem. Res. 2017, 50, 2085. - PubMed
- a) Meyer E. E., Rosenberg K. J., Israelachvili J., Proc. Natl. Acad. Sci. USA 2006, 103, 15739; - PMC - PubMed
- b) Aggeli A., Bell M., Carrick L. M., Fishwick C. W. G., Harding R., Mawer P. J., Radford S. E., Strong A. E., Boden N., J. Am. Chem. Soc. 2003, 125, 9619; - PubMed
- c) Paramonov S. E., Jun H.‐W., Hartgerink J. D., J. Am. Chem. Soc. 2006, 128, 7291; - PubMed
- d) Hu Y., Lin R., Zhang P., Fern J., Cheetham A. G., Patel K., Schulman R., Kan C., Cui H., ACS Nano 2016, 10, 880; - PubMed
- e) Zhou J., Du X., Gao Y., Shi J., Xu B., J. Am. Chem. Soc. 2014, 136, 2970; - PMC - PubMed
- f) Wang J., Liu K., Yan L., Wang A., Bai S., Yan X., ACS Nano 2016, 10, 2138; - PubMed
- g) Sawada T., Yamagami M., Ohara K., Yamaguchi K., Fujita M., Angew. Chem., Int. Ed. 2016, 55, 4519; - PubMed
- h) Sawada T., Matsumoto A., Fujita M., Angew. Chem., Int. Ed. 2014, 53, 7228. - PubMed
- a) Ren C., Zhang J., Chen M., Yang Z., Chem. Soc. Rev. 2014, 43, 7257; - PubMed
- b) Linna A., Gilani M. R. H. S., Gaolin L., Curr. Med. Chem. 2014, 21, 2453; - PubMed
- c) Webber M. J., Appel E. A., Meijer E. W., Langer R., Nat. Mater. 2016, 15, 13; - PubMed
- d) Cheetham A. G., Chakroun R. W., Ma W., Cui H., Chem. Soc. Rev. 2017, 46, 6638; - PMC - PubMed
- e) Wei G., Su Z., Reynolds N. P., Arosio P., Hamley I. W., Gazit E., Mezzenga R., Chem. Soc. Rev. 2017, 46, 4661. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous