Effect of fluid dynamics on decellularization efficacy and mechanical properties of blood vessels - PubMed (original) (raw)
Effect of fluid dynamics on decellularization efficacy and mechanical properties of blood vessels
Robin Simsa et al. PLoS One. 2019.
Abstract
Decellularization of blood vessels is a promising approach to generate native biomaterials for replacement of diseased vessels. The decellularization process affects the mechanical properties of the vascular graft and thus can have a negative impact for in vivo functionality. The aim of this study was to determine how detergents under different fluid dynamics affects decellularization efficacy and mechanical properties of the vascular graft. We applied a protocol utilizing 1% TritonX, 1% Tributyl phosphate (TnBP) and DNase on porcine vena cava. The detergents were applied to the vessels under different conditions; static, agitation and perfusion with 3 different perfusion rates (25, 100 and 400 mL/min). The decellularized grafts were analyzed with histological, immunohistochemical and mechanical tests. We found that decellularization efficacy was equal in all groups, however the luminal ultrastructure of the static group showed remnant cell debris and the 400 mL/min perfusion group showed local damage and tearing of the luminal surface. The mechanical stiffness and maximum tensile strength were not influenced by the detergent application method. In conclusion, our results indicate that agitation or low-velocity perfusion with detergents are preferable methods for blood vessel decellularization.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: The author R. Simsa is employed as an industrial PhD student at the company VERIGRAFT AB, which seeks to commercialize tissue engineering products, including products that underwent decellularization processes. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
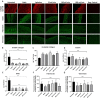
Fig 1. Schematic illustration of the decellularization setup.
Porcine vena cavas were decellularized with detergents (1) under static conditions, (2) by agitation at 115 rpm or (3–5) by perfusion, with agitation at 115 rpm and perfusion at 25, 100 or 400 mL/min.
Fig 2. Analysis of remaining DNA content following decellularization.
Porcine vena cavas were decellularized with an established protocol utilizing TX, TnBP and DNase. (A) Remaining DNA content in the tissue was measured. Result are shown as DNA in ng per mg wet tissue (n = 5 for all groups). Y-axis uses a logarithmic scale. (B) Whole-mount tissue sections were stained with DAPI and nuclei were visualized under a fluorescence microscope. Scale bar = 100 μm.
Fig 3. DAPI and H&E of decellularized vessels.
Porcine vena cavas were decellularized with TX, TnBP and DNase. Cross sections of paraffin-embedded samples were stained with DAPI (upper panels, nuclei are white) and H&E (lower panels, nuclei are blue). Scale bar = 50 μm.
Fig 4. Presence of ECM proteins collagen, elastin, fibronectin, vitronectin and GAGs following decellurarization.
Porcine vena cavas were decellularized and analyzed for ECM proteins. (A) Cross-section of paraffin embedded samples were immunostained for collagen type I/III, elastin, fibronectin and vitronectin. Images were taken at identical microscopy settings. Scale bar is 100 μm. (B-E) Quantification of ECM components with commercially available kits from 20–30 mg wet tissue (B) soluble collagen, (C) insoluble collagen, (D) elastin, and (E) GAGs. Fluorescence intensity of samples immunostained for (F) fibronectin and (G) vitronectin. The fluoresvence intensity was quantified with ImageJ, and the values were normalized to the untreated group. n = 5 for all groups.
Fig 5. Scanning electron microscopy analysis of porcine vena cava following decellularization.
Porcine vena cavas were decellularized and biopsies with a 4 mm diameter were punched out and prepared for SEM analyses. 500x magnification (scale bar = 50 μm), insets show 2500x magnification (scale bar = 10 μm). Two images (from two different samples) for 400 mL/min group show local tearing and flattening of surface to various degrees at different sample areas.
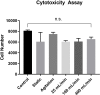
Fig 6. Cytotoxicity of ECM samples.
Equally sized ECM pieces were added to HUVECs seeded in a 96-well plate and cell number was measured with MTS assay 48h after incubation. Control consists of cells without added ECM pieces. n = 5 in duplicates for all groups.
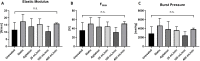
Fig 7. Mechanical testing of untreated and decellularized porcine vena cava.
Mechanical testing was performed on equally sized vessel ringlets placed on metal U-holders in a Zwick Roell testing machine. The vessels ringlets were stretched until failure and stress-strain curves were recorded. n = 5 vessels for all groups, 3 ringlets per vessel tested as technical replicates. (A) Elastic Modulus (Youngs Modulus) calculated from linear phase of stress strain curve. Higher values indicates increased stiffness and lower values indicates increased elasticity. (B) Fmax values (maximum tensile strength). (C) Theoretical burst pressure calculated from vessel properties and Fmax with Barlows equation.
Similar articles
- Systematic in vitro comparison of decellularization protocols for blood vessels.
Simsa R, Padma AM, Heher P, Hellström M, Teuschl A, Jenndahl L, Bergh N, Fogelstrand P. Simsa R, et al. PLoS One. 2018 Dec 17;13(12):e0209269. doi: 10.1371/journal.pone.0209269. eCollection 2018. PLoS One. 2018. PMID: 30557395 Free PMC article. - Combination of freeze-thaw with detergents: A promising approach to the decellularization of porcine carotid arteries.
Cheng J, Wang C, Gu Y. Cheng J, et al. Biomed Mater Eng. 2019;30(2):191-205. doi: 10.3233/BME-191044. Biomed Mater Eng. 2019. PMID: 30741667 - Fast, robust and effective decellularization of whole human livers using mild detergents and pressure controlled perfusion.
Willemse J, Verstegen MMA, Vermeulen A, Schurink IJ, Roest HP, van der Laan LJW, de Jonge J. Willemse J, et al. Mater Sci Eng C Mater Biol Appl. 2020 Mar;108:110200. doi: 10.1016/j.msec.2019.110200. Epub 2019 Sep 12. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 31923991 - The Emerging Role of Decellularized Plant-Based Scaffolds as a New Biomaterial.
Harris AF, Lacombe J, Zenhausern F. Harris AF, et al. Int J Mol Sci. 2021 Nov 16;22(22):12347. doi: 10.3390/ijms222212347. Int J Mol Sci. 2021. PMID: 34830229 Free PMC article. Review. - Human Umbilical Vessels: Choosing the Optimal Decellularization Method.
Rodríguez-Rodríguez VE, Martínez-González B, Quiroga-Garza A, Reyes-Hernández CG, de la Fuente-Villarreal D, de la Garza-Castro O, Guzmán-López S, Elizondo-Omaña RE. Rodríguez-Rodríguez VE, et al. ASAIO J. 2018 Sep/Oct;64(5):575-580. doi: 10.1097/MAT.0000000000000715. ASAIO J. 2018. PMID: 29095734 Review.
Cited by
- Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering.
Zhang X, Chen X, Hong H, Hu R, Liu J, Liu C. Zhang X, et al. Bioact Mater. 2021 Sep 23;10:15-31. doi: 10.1016/j.bioactmat.2021.09.014. eCollection 2022 Apr. Bioact Mater. 2021. PMID: 34901526 Free PMC article. Review. - Biomimetic Approaches in Scaffold-Based Blood Vessel Tissue Engineering.
Rosellini E, Giordano C, Guidi L, Cascone MG. Rosellini E, et al. Biomimetics (Basel). 2024 Jun 22;9(7):377. doi: 10.3390/biomimetics9070377. Biomimetics (Basel). 2024. PMID: 39056818 Free PMC article. Review. - The Potential of a New Natural Vessel Source: Decellularized Intercostal Arteries as Sufficiently Long Small-Diameter Vascular Grafts.
Xia Y, Zhou H, Ou JS, Liu Y. Xia Y, et al. Bioengineering (Basel). 2024 Jul 10;11(7):700. doi: 10.3390/bioengineering11070700. Bioengineering (Basel). 2024. PMID: 39061783 Free PMC article. - Decellularization in Tissue Engineering and Regenerative Medicine: Evaluation, Modification, and Application Methods.
Neishabouri A, Soltani Khaboushan A, Daghigh F, Kajbafzadeh AM, Majidi Zolbin M. Neishabouri A, et al. Front Bioeng Biotechnol. 2022 Apr 25;10:805299. doi: 10.3389/fbioe.2022.805299. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35547166 Free PMC article. Review. - Increased efficiency of peripheral nerve regeneration using supercritical carbon dioxide-based decellularization in acellular nerve graft.
Choi SJ, Han J, Shin YH, Kim JK. Choi SJ, et al. Sci Rep. 2024 Oct 10;14(1):23696. doi: 10.1038/s41598-024-72672-w. Sci Rep. 2024. PMID: 39389997 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
This project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No 722779 and was conducted within the “Training 4 Cell Regenerative Medicine” (T4CRM) network.
LinkOut - more resources
Full Text Sources