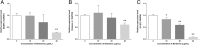Berberine inhibits NLRP3 Inflammasome pathway in human triple-negative breast cancer MDA-MB-231 cell - PubMed (original) (raw)
Berberine inhibits NLRP3 Inflammasome pathway in human triple-negative breast cancer MDA-MB-231 cell
Mingjiang Yao et al. BMC Complement Altern Med. 2019.
Abstract
Background: Breast cancer is still the most common malignant tumor that threatens the female's life in the world, especially triple-negative breast cancer (TNBC), one of the most difficult subtypes. Lack of targeted therapies brings about urgent demand for novel treatments. In this study we aim to investigate the anti-tumor activity of Berberine (BBR), a Chinese plant-derived alkaloid, against the TNBC cell line MDA-MB-231 and elucidate its mechanism referring to anti-inflammation.
Methods: Cell inhibition rate was measured by Cell Proliferation Assay, the cytotoxic effects was detected by Lactate dehydrogenase (LDH) leakage assay, the colony formation and migration potential were evaluated by colony formation assay and wound healing assay, the release of inflammatory cytokines was detected by EMD multifactor detection, and alterations of proteins and genes related to the NLR family pyrin domain containing 3 (NLRP3) inflammasome pathway were analyzed using western blotting and real-time Polymerase Chain Reaction (PCR).
Results: BBR reduce the viability of MDA-MB-231 cells and increased the release of LDH from the cells in a dose-dependent manner, with and inhibition of colony formation potential and migration of the cells. BBR also caused a marked reduction in the secretion of proinflammatory cytokines, Interleukin-1α (IL-1α), Interleukin-1β (IL-1β), Interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α). Besides, a down-regulated behavior was observed with the expression of P2X purinoceptor 7 (P2X7), NLRP3, pro-caspase-1, apoptosis-associated speck-like protein containing a caspase-activation and recruitment domain (ASC), caspase-1 p20, Interleukin-18 (IL-18), IL-1β proteins and NLRP3, Caspase-1 and ASC mRNAs in the NLRP3 inflammasome cascade.
Conclusions: Our results confirmed that BBR can effectively affect both tumor outgrowth and spontaneous metastasis in TNBC, and that we identified a new mechanism associated with inhibition the NLRP3 inflammasome pathway, suggesting its potential therapeutic relevance in clinical use.
Keywords: Anti-inflammation; Berberine; NLRP3 inflammasome; Triple-negative breast cancer.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Fig. 1
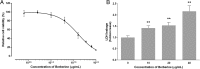
Effect of BBR on MDA-MB-231 viability and LDH release. Cells were seeded in 96-well plates at 5000 cells per well and treated with various concentrations of BBR for 48 h, the control wells were treated with equivalent amount of medium alone. The effect of BBR on MDA-MB-231 (a) viability and (b) LDH release was determined by the assays described in the Methods section. The relative cell viability and LDH leakage was calculated as the ratio of the absorbance at 490 nm of each treatment group against those of the corresponding untreated control group. Each value represents the mean ± S.D. (n = 6) of three independent experiments. * p < 0.05, ** p < 0.01, compared to the control
Fig. 2
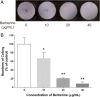
Effect of BBR on colony formation potential of MDA-MB-231 cells. Cells were seeded in 6-well plates at 1000 cells per well following treatment with indicated concentrations of BBR for 24 h. The medium was then replaced with fresh media, and the cells were allowed to grow for 12 days at 37 °C in a humidified atmosphere (5% CO2 in air) before staining with Giemsa stain solution. a Typical results of the clonogenic assay. b The surviving fraction of treated MDA-MB-231 cell line is presented as mean ± S.D. (n = 3) of three independent experiments. * p < 0.05, ** p < 0.01, compared to the control
Fig. 3
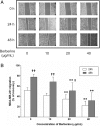
Effect of BBR on MDA-MB-231 cell migration. Cells were seeded into 12-well plates at 1 × 105 cells per well and cultured to near confluence. The wounded monolayer was incubated in culture medium containing various concentrations of BBR for 24 and 48 h. a Representative images of wound healing assays for cells treated with various concentrations of BBR, for 24 and 48 h. b The percent recovery as determined in the scratch wound healing assay. Data are presented as the mean ± S.D. (n = 3) of three independent experiments. *, p < 0.01, compared to the control; †, p < 0.05, †† p < 0.01, compared to 24 h, respectively
Fig. 4
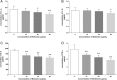
Effect of BBR on inflammatory cytokines in the supernatant of MDA-MB-231 cells. Cells were seeded into 12-well plates at 1 × 105 cells per well following treatment with indicated concentrations of BBR for 48 h, and the supernatant of MDA-MB-231 cells was collected and the cytokines (a) IL-1α, (b) IL-1β, (c) IL-6, and (d) TNF-α were analyzed. Data were presented as mean ± S.D. of six duplicated wells. * p < 0.05, ** p < 0.01, compared to the control
Fig. 5
Expression profile of NLRP3 inflammasome related gene in MDA-MB-231 cells treated with BBR. After treatment with various concentrations of BBR for 48 h, the expression profile of NLRP3 inflammasome related gene, (a) NLRP3, (b) Caspase-1, and (c) IL-1β, was analyzed using real-time PCR. Results are shown as the mean ± S.D. from three independent experiments. Significant difference between control and treatment with BBR are shown, * p < 0.05, ** p < 0.01
Fig. 6
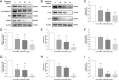
Effects of BBR on the expression profile of NLRP3 inflammasome signaling proteins in MDA-MB-231 cells. After treatment with various concentrations of BBR for 48 h, the protein bands (a) and (b), as well as expression profile of NLRP3 inflammasome signaling proteins, (c) P2X7, (d) pro-caspase1, (e) ASC, (f) NLRP3, (g) caspase-1 p20, (h) IL-1β and (i) IL-18, was analyzed using western blot as described in Material and methods. Significant difference between control and treatment with BBR are shown, * p < 0.05, ** p < 0.01. Representative image of the expression profile of each protein is shown from three independent experiments
Similar articles
- Activation of NLRP3 inflammasome by lymphocytic microparticles via TLR4 pathway contributes to airway inflammation.
Qiu Q, Yang Z, Cao F, Yang C, Hardy P, Yan X, Yang S, Xiong W. Qiu Q, et al. Exp Cell Res. 2020 Jan 15;386(2):111737. doi: 10.1016/j.yexcr.2019.111737. Epub 2019 Nov 20. Exp Cell Res. 2020. PMID: 31759058 - Aloe vera downregulates LPS-induced inflammatory cytokine production and expression of NLRP3 inflammasome in human macrophages.
Budai MM, Varga A, Milesz S, Tőzsér J, Benkő S. Budai MM, et al. Mol Immunol. 2013 Dec;56(4):471-9. doi: 10.1016/j.molimm.2013.05.005. Epub 2013 Aug 1. Mol Immunol. 2013. PMID: 23911403 - L5-LDL from ST-elevation myocardial infarction patients induces IL-1β production via LOX-1 and NLRP3 inflammasome activation in macrophages.
Yang TC, Chang PY, Lu SC. Yang TC, et al. Am J Physiol Heart Circ Physiol. 2017 Feb 1;312(2):H265-H274. doi: 10.1152/ajpheart.00509.2016. Epub 2016 Nov 18. Am J Physiol Heart Circ Physiol. 2017. PMID: 27864235 - Mechanism and Regulation of NLRP3 Inflammasome Activation.
He Y, Hara H, Núñez G. He Y, et al. Trends Biochem Sci. 2016 Dec;41(12):1012-1021. doi: 10.1016/j.tibs.2016.09.002. Epub 2016 Sep 23. Trends Biochem Sci. 2016. PMID: 27669650 Free PMC article. Review. - [Effect of NLRP3 inflammasome on vascular diseases].
Cao Z, Li Y, Chen R, Zeng P. Cao Z, et al. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2016 Nov 28;41(11):1232-1236. doi: 10.11817/j.issn.1672-7347.2016.11.020. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2016. PMID: 27932773 Review. Chinese.
Cited by
- Nutraceutical Compounds Targeting Inflammasomes in Human Diseases.
Castejón-Vega B, Giampieri F, Alvarez-Suarez JM. Castejón-Vega B, et al. Int J Mol Sci. 2020 Jul 8;21(14):4829. doi: 10.3390/ijms21144829. Int J Mol Sci. 2020. PMID: 32650482 Free PMC article. Review. - Suppression of inflammation-induced lung cancer cells proliferation and metastasis by exiguaflavanone A and exiguaflavanone B from Sophora exigua root extract through NLRP3 inflammasome pathway inhibition.
Arjsri P, Srisawad K, Semmarath W, Umsumarng S, Rueankham L, Saiai A, Rungrojsakul M, Katekunlaphan T, Anuchapreeda S, Dejkriengkraikul P. Arjsri P, et al. Front Pharmacol. 2023 Nov 10;14:1243727. doi: 10.3389/fphar.2023.1243727. eCollection 2023. Front Pharmacol. 2023. PMID: 38026959 Free PMC article. - Berberine a traditional Chinese drug repurposing: Its actions in inflammation-associated ulcerative colitis and cancer therapy.
Zhu C, Li K, Peng XX, Yao TJ, Wang ZY, Hu P, Cai D, Liu HY. Zhu C, et al. Front Immunol. 2022 Dec 6;13:1083788. doi: 10.3389/fimmu.2022.1083788. eCollection 2022. Front Immunol. 2022. PMID: 36561763 Free PMC article. Review. - Apoptosis Induction, a Sharp Edge of Berberine to Exert Anti-Cancer Effects, Focus on Breast, Lung, and Liver Cancer.
Zhu Y, Xie N, Chai Y, Nie Y, Liu K, Liu Y, Yang Y, Su J, Zhang C. Zhu Y, et al. Front Pharmacol. 2022 Jan 27;13:803717. doi: 10.3389/fphar.2022.803717. eCollection 2022. Front Pharmacol. 2022. PMID: 35153781 Free PMC article. Review. - Tumor microenvironment: a prospective target of natural alkaloids for cancer treatment.
Luo Y, Yin S, Lu J, Zhou S, Shao Y, Bao X, Wang T, Qiu Y, Yu H. Luo Y, et al. Cancer Cell Int. 2021 Jul 20;21(1):386. doi: 10.1186/s12935-021-02085-6. Cancer Cell Int. 2021. PMID: 34284780 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous