The Therapeutic Implications of Tea Polyphenols Against Dopamine (DA) Neuron Degeneration in Parkinson's Disease (PD) - PubMed (original) (raw)
The Therapeutic Implications of Tea Polyphenols Against Dopamine (DA) Neuron Degeneration in Parkinson's Disease (PD)
Zhi Dong Zhou et al. Cells. 2019.
Abstract
: Accumulative evidence indicated that the pathologically accumulated metal ions (iron species and Mn3+) and abnormally up-regulated monoamine oxidase B (MAOB) activity induced oxidation of endogenous dopamine (DA) can lead to mitochondria impairment, lysosome dysfunction, proteasome inhibition, and selective DA neuron vulnerability, which is implicated in the pathogenesis of Parkinson's disease (PD). The DA oxidation can generate deleterious reactive oxygen species (ROS) and highly reactive DA quinones (DAQ) to induce DA-related toxicity, which can be alleviated by DA oxidation suppressors, ROS scavengers, DAQ quenchers, and MAOB inhibitors. On the other hand, the nuclear factor erythroid 2-related factor 2 (Nrf2)-Keap1 and Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) anti-oxidative and proliferative signaling pathways play roles in anti-oxidative cell defense and mitochondria biogenesis, which is implicated in DA neuron protections. Therefore, agents with capabilities to suppress DA-related toxicity including inhibition of DA oxidation, scavenge of ROS, detoxification of DAQ, inhibition of MAOB, and modulations of anti-oxidative signaling pathways can be protective to DA neurons. Accumulative evidence shows that tea or coffee consumptions and smoking are related to deceased PD prevalence with unknown mechanisms. In this study, we investigate the protective capabilities of tea polyphenols and other PD relevant agents to inhibit DA-related toxicity and protect against environmental or genetic factors induced DA neuron degeneration in vitro and in vivo. We find that tea polyphenols can significantly suppress DA-related toxicity to protect DA neurons. The tea polyphenols can protect DA neurons via inhibition of DA oxidation, conjugation with DAQ, scavenge of ROS, inhibition of MAOB, and modulations of Nrf2-Keap1 and PGC-1α anti-oxidative signaling pathways. The tea polyphenols with more phenolic hydroxyl groups and ring structures have stronger protective functions. The protective capabilities of tea polyphenols is further strengthened by evidence that phenolic hydroxyl groups can directly conjugate with DAQ. However, GSH and other sulfhydyl groups containing agents have weaker capabilities to abrogate DA oxidation, detoxify ROS and DAQ and inhibit MAOB; whereas nicotine (NICO) and caffeine (CAF) can only modulate Nrf2-Keap1 and PGC-1α pathways to protect DA neurons weakly. The tea polyphenols are identified to protect against overexpression of mutant A30P α-synuclein (α-syn) induced DA neuron degeneration and PD-like symptoms in transgenic Drosophila. Based on achievements from current studies, the excellent and versatile protective capabilities of tea polyphenols are highlighted, which will contribute and benefit to future anti-PD therapy.
Keywords: Parkinson’s disease; anti-PD therapy; dopamine; neurodegeneration; polyphenols.
Conflict of interest statement
Supplementary Materials: The following are available online at www.mdpi.com/xxx/s1, Figure S1: Molecular structures of tea polyphenols and other agents, Figure S2: Impacts of various agents on cell viability of PC12 cells, Figure S3: Vector constructive map of ARE-pGL3promoter vector, Figure S4: HPLC and LC-MS-MS analysis of 3 peptides synthesized.
Figures
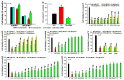
Figure 1
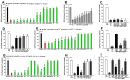
Protections against factors induced dopamine (DA) oxidation by tea polyphenols and other agents. The oxidation of 100 μM DA is induced by 400 μM Mn3+, 200 μM Fe3+ and 10-unit Tyro respectively for 3 min at room temperature in the presence or absence of various agents before HPLC analysis of DA content in solutions. Freshly prepared DA is set as control and DA peak areas are expressed as % control. *, at least p < 0.05, compared with DA peak areas of controls. #, at least p < 0.05, compared with peak areas of DA after Mn3+, Fe3+ and Tyro induced DA oxidation respectively in the absence of protective agents. (A–D), Protection against Mn3+ induced DA oxidation by different agents. (A) Protection by 1 mM agents. (B) Dosage dependent protection by L-cys. (C) glutathione (GSH) and N-acetyle-cysteine (NAC) cannot protect against Mn3+ induced DA oxidation. (D) Protection by 250 μM tea polyphenols. (E) Protection against Fe3+ induced DA oxidation by 1 mM agents. (F–I), Protection against tyrosinase induced DA oxidation by various agents. (F) Dosage dependent protection by CAF. (G) Protection by in 1 mM agents. (H) Protections by 100 μM tea polyphenols. (I) Dosage dependent protection by epigallocatechin (EGC) and GA.
Figure 2
Detections of reductive potency of tea polyphenols and other agents. The reductive potencies of tea polyphenols and various Parkinson’s disease (PD)-related agents were detected by 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) cation decolorization assay. Solutions in the absence of polyphenols and other agents are set as controls. The reductive potency of various agents at different concentration is expressed as % control of absorbance at 734 nm in ABTS cation decolorization reactions. *, at least p < 0.05, compared with the absorbance at 734 nm of controls. #, at least p < 0.05, compared with the absorbance at 734 nm of the same agents at lower concentration. ^, at least p < 0.05, compared with the absorbance at 734 nm of GSH at the same concentration. (A) Analysis of reductive potency of GSH, NICO, CAF, MAN, VC and VE at 0.5 and 1 mM dosage. (B) Dosage dependent reductive potency of GSH, VC and VE. (C) Dosage dependent reductive potency of GSH, NAC and L-cys. (D) Analysis of reductive potency of GSH and tea polyphenols.
Figure 3
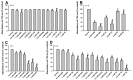
Inhibitions of monoamine oxidase B (MAOB) activity by tea polyphenols and other agents. MAOB activities of human dopaminergic SH-SY5Y cell lysates in the presence of MAOB inhibitors and tea polyphenols as well as other agents are detected. MAOB activity of cell lysates in the absence of MAOB inhibitor and other agents is set as controls. MAOB activity of cell lysates in the presence of MABO inhibitors or other agents is expressed as % control. *, at least p < 0.05, compared with MAOB activity of controls. (A) MAOB inhibition by 10 and 200 μM caffeine (CAF), nicotine (NICO), mannitol (MAN), α-Tocopherol (VE), ascorbic acid (VC), GSH, NAC, and L-cys. (B) MAOB inhibition by 10 and 50 μM tea polyphenols. (C) rasagiline (RA) and pargyline (PA) induced MAOB inhibition.
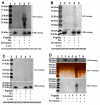
Figure 4
Modulations of Nrf2-Keap1 and PGC-1α signaling pathways by tea polyphenols and other agents. (A–C), potential modulations of nuclear factor erythroid 2-related factor 2 (Nrf2)-Keap1 and Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) signaling pathways by tea polyphenols and other agents are monitored in antioxidant response element (ARE)-Luciferase and PGC-1α promoter luciferase vectors transfected HEK cells respectively after overnight treatment by various agents. Cells were lyzed and luciferase activities are analyzed. (A–C), monitoring of agents induced modulation of Nrf2-keap1 or PGC-1α signaling pathways by luciferase assay. *, p < 0.001, compared with the luciferase value of control cells. (A) Modulation of Nrf2-keap1 pathway by GSH, Mann, VE, VC, NAC, and
l
-cys. (B) Modulation Nrf2-keap1 pathway by NICO and CAF. (C) Monitoring of NICO and CAF induced modulation of PGC-1α pathway. (D–H) Agents induced influence on Nrf2-Keap1 and PGC-1α signaling pathways in SH-SY5Y dopaminergic cells. (D–F) Representative western blot gel picture of HO-1, NQO-1, and PGC-1α protein bands in the presence or absence of NICO or CAF. (D), HO-1 protein bands under NICO treatment; (E) NQO-1 protein bands under CAF treatment; (F), PGC-1α protein bands under CAF and NICO treatments. (G,H) Quantitative analysis of NICO or CAF induced up-regulated expressions of HO-1 and NQO-1 (G) and PGC-1α (H), based on densitometric scanning of protein bands in Western blot gels. *, at least p < 0.05, compared with the densitometric value of protein bands of cells without NICO or CAF treatments. (I,J), monitoring of 6 hr polyphenols treatment induced modulation of Nrf2-keap1 (I) or PGC-1α (J) signaling pathways by luciferase assay. *, at least p < 0.05, compared with the luciferase value of control cells. #, at least p < 0.01, compared with the luciferase value of cells transfected with mutant luciferase vectors (K,L), polyphenols induced modulations of Nrf2-Keap1 and PGC-1α signaling pathways in SH-SY5Y dopaminergic cells, validated by Western blot analysis. (K) Representative western blot gel picture of HO-1 and PGC-1α protein bands after 6 h treatments by GA, EGG and TA, (L) quantitative analysis data based on densitometric scanning of HO-1 and PGC-1α protein bands in western blot gels. *, at least p < 0.05, compared with the respective densitometric value of HO-1 and PGC-1α protein bands of cells without polyphenols treatments. (M) and (N), EGC, EGCG, and TF fail to modulate Nrf2-Keap1 and PGC-1α signaling pathways in SH-SY5Y dopaminergic cells. (M) Representative western blot gel picture of HO-1 and PGC-1α protein bands after 6 hr treatments by EGC, EGC, and TF, (N), quantitative analysis data based on densitometric scanning of HO-1 and PGC-1α protein bands in western blot gels.
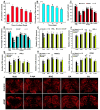
Figure 5
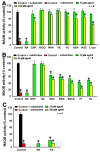
Protection against Mn3+, H2O2, and AM induced dopaminergic PC12 cell death by tea polyphenols and other agents. Dopaminergic PC12 cells were challenges with 200 or 300 μM Mn3+ or 300 μM H2O2 overnight or 100 μM AM 3 h respectively in the presence or absence of various agents. Cells without any challenges are set as control. *, at least p < 0.05, compared with cell viability of control cells. #, at least p < 0.05, compared with cell viability of cells challenged with stressors only. (A,B), The Mn3+ induced cell toxicity are dependent on endogenous DA level in PC12 cells. (A) Influence on PC12 cell viability by tyrosine hydroxylase (TH) overexpression or knockdown under Mn3+ overnight challenge. PC12 cells were transfected with rat-TH or TH shRNA vectors overnight respectively, before subsequent 200 μM Mn3+ overnight challenge. (B) Influence on DA level in PC12 cells by TH overexpression or knockdown. (C–E) Protection of PC12 cells against 300 μM Mn3+ overnight challenges induced toxicity by different agents. (C) Protection by 50 and 500 μM CAF, NICO, MAN, VE, VC, GSH, and
l
-cys. (D) Protection by 25 and 100 μM GA, EGC, EGCG, TF, and TA. (E) Protection by 250 μM GSH,
l
-cys and tea polyphenols. (F–H), protection against 300 μM H2O2 or 100 μM AM challenges induced toxicity by various agents. (F) Protection against H2O2 induced toxicity by 250 and 1000 μM CAF, NICO, and MAN. (G) Protection against H2O2 induced toxicity by 250 μM agents. (H) Protection against 100 μM AM induced toxicity by 250 μM agents.
Figure 6
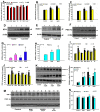
Conjugations of DA quinones (DAQ) to phenolic hydroxyl groups of synthesized peptides. Three peptides synthesized react with DA under tyrosinase catalysis in the presence or absence of
l
-cys. After reaction, peptides are precipitated and analyzed by SDS-PAGE, visualized by nitroblue tetrazolium (NBT), silver, and CBB R-250 staining respectively. (A) Peptide Y with 5 phenolic hydroxyl groups (tyrosine residues) can react with DAQ well in solutions, (B) peptide S with 5 non-phenolic hydroxyl groups (serine residues) have poor capability to react with DAQ. (C) Peptides G with no hydroxyl groups (glycine residues) have poor capability to react with DAQ in solutions. (D) Comparison of reactions capability of 3 peptides with DAQ.
Figure 7
Protection against overexpression of mutant A30P α-synuclein (α-syn) induced DA neuron degeneration in fly head by GA, TA, NAC, and
l
-cys. Yellow white control or transgenic α-syn A30P mutant flies were crossed with ddc-GAL4 lines to induce overexpression of A30P mutant α-syn specifically in DA neurons in transgenic fly heads. Flies were cultured for 30 days in the presence or absence of 2 mM GA, TA, NAC, and
l
-cys before check of fly climbing behavior and analysis of DA content and TH positive DA neuron numbers in fly heads. Flies without any drug treatments are set as void group. *, at least p < 0.05, compared with yellow white void flies without any drug treatment. (A,B), age dependent decrease of DA contents (A) and impairment of climbing capabilities (B) of yellow white files; (C) HPLC analysis of DA contents in transgenic fly heads; (F), monitoring climbing capabilities of transgenic flies. (E–I), numbers of TH positive DA neurons in different districts of transgenic fly heads. (E), PAL; (F), PPL1; (G), PPL2; (H), PPM1/2; (I), PPM3. (J) Confocal fluorescent images of TH positive DA neurons in transgenic fly heads with or without treatments by various agents.
Similar articles
- Neuroprotective effect of damaurone D in a C. elegans model of Parkinson's disease.
Lee SH, Han YT, Cha DS. Lee SH, et al. Neurosci Lett. 2021 Mar 16;747:135623. doi: 10.1016/j.neulet.2021.135623. Epub 2021 Jan 19. Neurosci Lett. 2021. PMID: 33482307 - PGC-1α activity in nigral dopamine neurons determines vulnerability to α-synuclein.
Ciron C, Zheng L, Bobela W, Knott GW, Leone TC, Kelly DP, Schneider BL. Ciron C, et al. Acta Neuropathol Commun. 2015 Apr 1;3:16. doi: 10.1186/s40478-015-0200-8. Acta Neuropathol Commun. 2015. PMID: 25853296 Free PMC article. - Disruption of dopamine homeostasis underlies selective neurodegeneration mediated by alpha-synuclein.
Park SS, Schulz EM, Lee D. Park SS, et al. Eur J Neurosci. 2007 Dec;26(11):3104-12. doi: 10.1111/j.1460-9568.2007.05929.x. Epub 2007 Nov 14. Eur J Neurosci. 2007. PMID: 18005066 - Cellular and molecular mechanisms of antioxidants in Parkinson's disease.
Sutachan JJ, Casas Z, Albarracin SL, Stab BR 2nd, Samudio I, Gonzalez J, Morales L, Barreto GE. Sutachan JJ, et al. Nutr Neurosci. 2012 May;15(3):120-6. doi: 10.1179/1476830511Y.0000000033. Nutr Neurosci. 2012. PMID: 22732354 Review. - Review on the interactions between dopamine metabolites and α-Synuclein in causing Parkinson's disease.
Sivakumar P, Nagashanmugam KB, Priyatharshni S, Lavanya R, Prabhu N, Ponnusamy S. Sivakumar P, et al. Neurochem Int. 2023 Jan;162:105461. doi: 10.1016/j.neuint.2022.105461. Epub 2022 Nov 30. Neurochem Int. 2023. PMID: 36460239 Review.
Cited by
- Vitamin B12 Ameliorates the Pathological Phenotypes of Multiple Parkinson's Disease Models by Alleviating Oxidative Stress.
Wu Y, Zhao Z, Yang N, Xin C, Li Z, Xu J, Ma B, Lim KL, Li L, Wu Q, Yu C, Zhang C. Wu Y, et al. Antioxidants (Basel). 2023 Jan 9;12(1):153. doi: 10.3390/antiox12010153. Antioxidants (Basel). 2023. PMID: 36671015 Free PMC article. - Parkinson's Disease Dementia: Synergistic Effects of Alpha-Synuclein, Tau, Beta-Amyloid, and Iron.
Han J, Fan Y, Wu P, Huang Z, Li X, Zhao L, Ji Y, Zhu M. Han J, et al. Front Aging Neurosci. 2021 Oct 11;13:743754. doi: 10.3389/fnagi.2021.743754. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34707492 Free PMC article. - Caffeine: An Overview of Its Beneficial Effects in Experimental Models and Clinical Trials of Parkinson's Disease.
Schepici G, Silvestro S, Bramanti P, Mazzon E. Schepici G, et al. Int J Mol Sci. 2020 Jul 4;21(13):4766. doi: 10.3390/ijms21134766. Int J Mol Sci. 2020. PMID: 32635541 Free PMC article. Review. - Independent and Joint Associations of Tea Consumption and Smoking with Parkinson's Disease Risk in Chinese Adults.
Nie J, Liu C, Yu C, Guo Y, Pei P, Yang L, Chen Y, Du H, Zhu K, Schmidt D, Avery D, Chen J, Chen Z, Lv J, Li L; China Kadoorie Biobank (CKB) Collaborative Group. Nie J, et al. J Parkinsons Dis. 2022;12(5):1693-1702. doi: 10.3233/JPD-223148. J Parkinsons Dis. 2022. PMID: 35527564 Free PMC article.
References
- Burbulla L.F., Song P., Mazzulli J.R., Zampese E., Wong Y.C., Jeon S., Santos D.P., Blanz J., Obermaier C.D., Strojny C., et al. Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science. 2017;357:1255–1261. doi: 10.1126/science.aam9080. - DOI - PMC - PubMed
- Biosa A., Arduini I., Soriano M.E., Giorgio V., Bernardi P., Bisaglia M., Bubacco L. Dopamine Oxidation Products as Mitochondrial Endotoxins, a Potential Molecular Mechanism for Preferential Neurodegeneration in Parkinson’s Disease. ACS Chem. Neurosci. 2018;9:2849–2858. doi: 10.1021/acschemneuro.8b00276. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous