Strong anion exchange-mediated phosphoproteomics reveals extensive human non-canonical phosphorylation - PubMed (original) (raw)
Strong anion exchange-mediated phosphoproteomics reveals extensive human non-canonical phosphorylation
Gemma Hardman et al. EMBO J. 2019.
Abstract
Phosphorylation is a key regulator of protein function under (patho)physiological conditions, and defining site-specific phosphorylation is essential to understand basic and disease biology. In vertebrates, the investigative focus has primarily been on serine, threonine and tyrosine phosphorylation, but mounting evidence suggests that phosphorylation of other "non-canonical" amino acids also regulates critical aspects of cell biology. However, standard methods of phosphoprotein characterisation are largely unsuitable for the analysis of non-canonical phosphorylation due to their relative instability under acidic conditions and/or elevated temperature. Consequently, the complete landscape of phosphorylation remains unexplored. Here, we report an unbiased phosphopeptide enrichment strategy based on strong anion exchange (SAX) chromatography (UPAX), which permits identification of histidine (His), arginine (Arg), lysine (Lys), aspartate (Asp), glutamate (Glu) and cysteine (Cys) phosphorylation sites on human proteins by mass spectrometry-based phosphoproteomics. Remarkably, under basal conditions, and having accounted for false site localisation probabilities, the number of unique non-canonical phosphosites is approximately one-third of the number of observed canonical phosphosites. Our resource reveals the previously unappreciated diversity of protein phosphorylation in human cells, and opens up avenues for high-throughput exploration of non-canonical phosphorylation in all organisms.
Keywords: mass spectrometry; non-canonical phosphorylation; phosphohistidine; phosphoproteomics; strong anion exchange chromatography.
© 2019 The Authors. Published under the terms of the CC BY 4.0 license.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
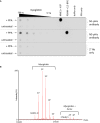
Figure EV1. Myoglobin is extensively phosphorylated on His following treatment with potassium phosphoramidate (PPA)
- PPA‐treated myoglobin preferentially generates N3‐phosphohistidine. Serial dilutions of myoglobin and PPA‐treated myoglobin were dotted onto nitrocellulose membrane and incubated with either the N1‐ or N3‐phosphohistidine antibody (or secondary antibody only), as indicated. Phosphorylated PGAM and NME1 proteins were also dotted onto the membrane, along with their unphosphorylated forms, as controls for the N1‐ and N3‐pHis antibodies, respectively. PGAM: phosphoglycerate mutase. NME1: nucleoside diphosphate kinase.
- Zero charge state mass spectrum of intact phosphorylated myoglobin. Phosphorylated myoglobin was analysed by direct infusion via nano‐ESI into a Synapt G2‐S_i_ mass spectrometer. The raw mass spectrum was deconvoluted using MaxEnt1. Up to five phosphate groups are observed per intact myoglobin molecule. Also apparent is the haem‐bound form of myoglobin containing up to four phosphate groups.
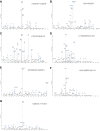
Figure EV2. Product ion spectra generated by HCD (unless stated) of pHis‐containing tryptic peptides from equine myoglobin
- A–G
The identified phosphorylation site is indicated (*), and the sequence is detailed on the mass spectrum. (A) doubly charged ion at m/z 843.9: pHis25; (B) doubly charged ion at m/z 676.3: pHis37; (C) doubly charged ion at m/z 729.9: pHis65; (D) triply charged ion at m/z 645.3: pHis82; (E) ETD spectrum of triply charged ion at m/z 645.3: pHis83; (F) triply charged ion at m/z 645.3: pHis94; and (G) triply charged ion at m/z 655.6: pHis114. The triplet neutral loss ions can be observed in all six HCD spectra (A–D, F–G) for pHis‐containing peptides from myoglobin.
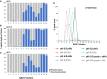
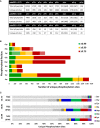
Figure 1. Optimal separation of pHis myoglobin peptides from their non‐phosphorylated counterparts by strong anion exchange (SAX) is achieved at pH 6.8
- A–C
Percentage of the total signal intensity attributed to phosphopeptides (blue bars, 11 unique peptide ions) and non‐phosphorylated peptides (grey bars, 25 unique peptide ions) following SAX fractionation at (A) pH 6.0, (B) pH 6.8 and (C) pH 8.0, with later fractions consisting of up to 100% phosphopeptide ion signal. - D
Representative pHis‐containing myoglobin peptide (LFTGHPETLEK, solid line) and its non‐phosphorylated counterpart (LFTGHPETLEK, dashed line), quantified across all 16 SAX fractions; SAX was performed at pH 6.0 (red), pH 6.8 (blue) or pH 8.0 (green). Percentage of the total peak area of each individual peptide across the gradient is plotted for each fraction. In order to assess pHis stability and effects of SAX separation in a complex mixture (black), SAX was also repeated at pH 6.8 with phosphorylated myoglobin spiked into a human cell lysate prior to digestion. Data for the five pHis‐containing myoglobin peptides are shown in Fig EV3.
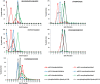
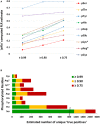
Figure EV3. Optimal separation of pHis myoglobin peptides from their non‐phosphorylated counterparts by strong anion exchange (SAX) is achieved at pH 6.8
Each of the five pHis‐containing myoglobin peptides (solid line) and their non‐phosphorylated counterparts (dashed line) were quantified across all 16 SAX fractions, where SAX was performed at pH 6 (red), pH 6.8 (blue) or pH 8 (green). Percentage of the total peak area of each individual peptide across the entire SAX separation is plotted for each fraction. SAX at pH 6.8 was also repeated with the phosphorylated myoglobin spiked into a human cell lysate prior to digestion in order to assess pHis stability and effects of SAX separation in a complex protein mixture (black). These data are representative of multiple repeat experiments. As can be seen (middle row, left panel), the non‐phosphorylated version of the peptide HGTVVLTALGGILK was quantified at ~50% in both fraction 1 and fraction 4 at pH 6.0, while the phosphorylated peptide is predominantly in fraction 3, confirming pHis hydrolysis during SAX chromatography at this pH. Similar observations were also made for the peptide LFTGHPEYLEK.
Figure 2. UPAX workflow for unbiased canonical and non‐canonical phosphoproteomics
- A
Schematic representation of the UPAX strategy for phosphopeptide enrichment and MS/MS analyses. - B
siRNA‐mediated knockdown of PHPT1 in HeLa cells (24 h) with reference to GAPDH loading control. NT—non‐targeting siRNA control. - C–F
(C) Representative SAX profile of trypsin‐digested HeLa lysate (Abs280 nm). Base peak chromatograms are shown for select SAX fractions following high‐resolution LC‐MS/MS using an Orbitrap Fusion mass spectrometer: (D) fraction 3 (green); (E) fraction 6 (red); and (F) fraction 10 (grey). Peptides were fragmented by HCD, with neutral loss of 98 amu from the precursor ions triggering EThcD (Ferries et al, 2017). Tandem mass spectra were separated according to fragmentation strategy in Proteome Discoverer prior to searching with Mascot. The ptmRS node was used for phosphosite localisation. Analysis was performed on three independent biological replicates for each condition (NT or PHPT1 siRNA).
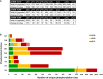
Figure 3. UPAX permits identification of extensive phosphorylation of canonical and non‐canonical phosphosites
- Total number of phosphopeptides identified (5% FDR) and unique sites for each phosphorylated residue according to site localisation confidence (ptmRS score). For pLys and pArg, the number in parentheses is the total number of identified sites/peptides including those localised to the peptide C‐terminus; outside of parentheses are the non‐C‐terminally mapped pLys or pArg sites.
- Number of unique phosphorylation sites defined at different site localisation confidence values: ptmRS ≥ 0.99 (green); ptmRS ≥ 0.90 (yellow); and ptmRS ≥ 0.75 (red). Light green, yellow or red indicates those sites of pLys or pArg mapped to the extreme peptide C‐terminal residue at each ptmRS score cut‐off.
- Percentage of canonical pSer, pThr and pTyr sites (shades of grey) compared with non‐canonical phosphosites (pHis—yellow; pAsp—blue; pGlu—green; pLys—red; pArg—purple) identified using either the described UPAX strategy or a standard TiO2‐based phosphopeptide enrichment protocol, as a function of ptmRS score.
Figure 4. A decoy pAla search can be used to estimate residue‐specific false localisation rate and thus the number of identified true‐positive phosphosites
- pAla‐computed FLR estimate for each type of canonical and non‐canonical phosphorylated residue at ptmRS values ≥ 0.99, ≥ 0.90 or ≥ 0.75.
- Total number of phosphorylation sites estimated to be “true positives” based on the pAla‐determined residue‐specific FLR, for each phosphorylated residue according to ptmRS score. pLys* and pArg* represent non‐C‐terminal pLys or pArg residues, respectively (see Appendix Supplementary Methods).
Figure EV4. Total numbers of identified phosphopeptides (1% FDR) and unique phosphosites for each phosphorylated residue according to site localisation confidence (_ptm_RS score)
- For pLys and pArg, the number in parentheses is the total number of identified sites/peptides including those localised to the peptide C‐terminus. Non‐C‐terminally mapped pLys or pArg sites are outside of the parentheses.
- The chart displays the total number of unique phosphorylation sites defined at different site localisation confidence values: _ptm_RS ≥0.99 (green); _ptm_RS ≥0.90 (yellow); and _ptm_RS ≥0.75 (red). Light green, yellow or red indicates those sites of pLys or pArg mapped to the extreme peptide C‐terminal residue at each _ptm_RS score cut‐off.
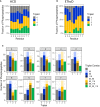
Figure 5. Phosphopeptide neutral loss pattern upon HCD is not diagnostic for phosphorylation of any given residue
- Comparison of triplet score (≥ 5% S/N) following HCD of unique phosphopeptides (5% FDR) identified from UPAX separated tryptic digests of HeLa cell‐derived phosphopeptides. Site of phosphorylation (ptmRS score ≥ 0.90) is indicated. Percentage of phosphopeptides exhibiting either no neutral loss (triplet = 0) or neutral loss of any 1 (triplet = 1), 2 (triplet = 2) or 3 ions from the precursor (∆80, ∆98 and/or ∆116 amu).
- Distribution of triplet score following HCD fragmentation as a function of site localisation confidence (ptmRS score ≥ 0.75, ≥ 0.90, ≥ 0.95 and ≥ 0.99) for unique singly phosphorylated peptide as a function of the residue phosphorylated.
- Numbers of phosphopeptides (ptmRS ≥ 0.90) exhibiting different combinations of the three neutral loss species (∆80, ∆98 and ∆116 amu from precursor) for each of the phosphorylated residues.
Figure 6. Motif analysis for pHis‐, pAsp‐ and pGlu‐containing peptides
- A–C
The amino acid sequences surrounding confidently localised sites of (A) pHis, (B) pAsp and (C) pGlu (ptmRS ≥ 0.99) were analysed for sequence enrichment using Motif‐X. Depicted are the sequences of the enriched motifs. Additional details are presented in Appendix Figs S8, S11 and S13.
Figure 7. Functional analysis of proteins containing either pAsp or pGlu
- A, B
Functional annotation of the significantly enriched (A) pAsp‐containing proteins or (B) pGlu‐containing proteins (ptmRS ≥ 0.90) using DAVID (_q_‐value < 0.05). BH: Benjamini–Hochberg.
Figure 8. Motif analysis for pLys‐, pArg‐ and pCys‐containing peptides
- A–C
The amino acid sequences surrounding confidently localised sites of (A) non‐C‐terminally localised pLys, (B) non‐C‐terminally localised pArg and (C) pCys (ptmRS ≥ 0.99) were analysed for sequence enrichment using Motif‐X. Depicted are the sequences of the enriched motifs. Additional details are presented in Appendix Figs S15, S17 and S18.
Similar articles
- High-Throughput Characterization of Histidine Phosphorylation Sites Using UPAX and Tandem Mass Spectrometry.
Hardman G, Eyers CE. Hardman G, et al. Methods Mol Biol. 2020;2077:225-235. doi: 10.1007/978-1-4939-9884-5_15. Methods Mol Biol. 2020. PMID: 31707662 - Rapid Shotgun Phosphoproteomics Analysis.
Carrera M, Cañas B, Lopez-Ferrer D. Carrera M, et al. Methods Mol Biol. 2021;2259:259-268. doi: 10.1007/978-1-0716-1178-4_17. Methods Mol Biol. 2021. PMID: 33687721 - Phosphopeptide enrichment using offline titanium dioxide columns for phosphoproteomics.
Yu LR, Veenstra T. Yu LR, et al. Methods Mol Biol. 2013;1002:93-103. doi: 10.1007/978-1-62703-360-2_8. Methods Mol Biol. 2013. PMID: 23625397 Free PMC article. - Tools for analyzing the phosphoproteome and other phosphorylated biomolecules: a review.
Leitner A, Sturm M, Lindner W. Leitner A, et al. Anal Chim Acta. 2011 Oct 3;703(1):19-30. doi: 10.1016/j.aca.2011.07.012. Epub 2011 Jul 19. Anal Chim Acta. 2011. PMID: 21843671 Review. - Targeted mass spectrometry: An emerging powerful approach to unblock the bottleneck in phosphoproteomics.
Osinalde N, Aloria K, Omaetxebarria MJ, Kratchmarova I. Osinalde N, et al. J Chromatogr B Analyt Technol Biomed Life Sci. 2017 Jun 15;1055-1056:29-38. doi: 10.1016/j.jchromb.2017.04.026. Epub 2017 Apr 17. J Chromatogr B Analyt Technol Biomed Life Sci. 2017. PMID: 28441545 Review.
Cited by
- Synthesis and Evaluation of Non-Hydrolyzable Phospho-Lysine Peptide Mimics.
Hauser A, Poulou E, Müller F, Schmieder P, Hackenberger CPR. Hauser A, et al. Chemistry. 2021 Feb 1;27(7):2326-2331. doi: 10.1002/chem.202003947. Epub 2020 Dec 7. Chemistry. 2021. PMID: 32986895 Free PMC article. - Differential Phospho-Signatures in Blood Cells Identify LRRK2 G2019S Carriers in Parkinson's Disease.
Garrido A, Santamaría E, Fernández-Irigoyen J, Soto M, Simonet C, Fernández M, Obiang D, Tolosa E, Martí MJ, Padmanabhan S, Malagelada C, Ezquerra M, Fernández-Santiago R. Garrido A, et al. Mov Disord. 2022 May;37(5):1004-1015. doi: 10.1002/mds.28927. Epub 2022 Jan 20. Mov Disord. 2022. PMID: 35049090 Free PMC article. - Considerations for defining +80 Da mass shifts in mass spectrometry-based proteomics: phosphorylation and beyond.
Daly LA, Clarke CJ, Po A, Oswald SO, Eyers CE. Daly LA, et al. Chem Commun (Camb). 2023 Sep 26;59(77):11484-11499. doi: 10.1039/d3cc02909c. Chem Commun (Camb). 2023. PMID: 37681662 Free PMC article. Review. - Extensive protein pyrophosphorylation revealed in human cell lines.
Morgan JAM, Singh A, Kurz L, Nadler-Holly M, Ruwolt M, Ganguli S, Sharma S, Penkert M, Krause E, Liu F, Bhandari R, Fiedler D. Morgan JAM, et al. Nat Chem Biol. 2024 Oct;20(10):1305-1316. doi: 10.1038/s41589-024-01613-5. Epub 2024 Apr 25. Nat Chem Biol. 2024. PMID: 38664588 Free PMC article. - In-depth and 3-dimensional exploration of the budding yeast phosphoproteome.
Lanz MC, Yugandhar K, Gupta S, Sanford EJ, Faça VM, Vega S, Joiner AMN, Fromme JC, Yu H, Smolka MB. Lanz MC, et al. EMBO Rep. 2021 Feb 3;22(2):e51121. doi: 10.15252/embr.202051121. Epub 2021 Jan 25. EMBO Rep. 2021. PMID: 33491328 Free PMC article.
References
- Attwood PV, Piggott MJ, Zu XL, Besant PG (2007) Focus on phosphohistidine. Amino Acids 32: 145–156 - PubMed
- Attwood PV, Besant PG, Piggott MJ (2011) Focus on phosphoaspartate and phosphoglutamate. Amino Acids 40: 1035–1051 - PubMed
- Attwood PV (2013) Histidine kinases from bacteria to humans. Biochem Soc Trans 41: 1023–1028 - PubMed
- Bentley‐DeSousa A, Downey M (2019) From underlying chemistry to therapeutic potential: open questions in the new field of lysine polyphosphorylation. Curr Genet 65: 57–64 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/M023818/1/UK Research and Innovation|Biotechnology and Biological Sciences Research Council (BBSRC)/International
- BB/L005239/1/UK Research and Innovation|Biotechnology and Biological Sciences Research Council (BBSRC)/International
- BB/N021703/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/H007113/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/M012557/1/UK Research and Innovation|Biotechnology and Biological Sciences Research Council (BBSRC)/International
- BB/R02216X/1/UK Research and Innovation|Biotechnology and Biological Sciences Research Council (BBSRC)/International
- CR1157/North West Cancer Research Fund (NWCR)/International
- BB/H007113/1/UK Research and Innovation|Biotechnology and Biological Sciences Research Council (BBSRC)/International
- BB/M025705/1/UK Research and Innovation|Biotechnology and Biological Sciences Research Council (BBSRC)/International
- CR1088/North West Cancer Research Fund (NWCR)/International
- CR1037/North West Cancer Research Fund (NWCR)/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials