Comparative assessment of methicillin resistant Staphylococcus aureus diagnostic assays for use in resource-limited settings - PubMed (original) (raw)
Comparative Study
Comparative assessment of methicillin resistant Staphylococcus aureus diagnostic assays for use in resource-limited settings
A Ayebare et al. BMC Microbiol. 2019.
Abstract
Background: The rise of methicillin-resistant Staphylococcus aureus (MRSA) is a global health concern. Paucity of data on MRSA carriage prevalence and diagnostic methods in resource-limited settings hampers efforts to define the problem and plan an appropriate response. Additionally, high variability in cost and logistical characteristics of MRSA screening methods may impede infection control efforts. We compared the performance of locally-available chromogenic agar BD CHROMagar MRSA II and two PCR-based assays (Hain GenoQuick MRSA and Cepheid Xpert SA Complete) for the detection of asymptomatic MRSA carriage in nasal swabs.
Results: During 2015, we enrolled 500 patients from five hospital wards at a Ugandan regional referral hospital. We found 30% prevalence of methicillin-sensitive Staphylococcus aureus (MSSA) nasal carriage, and 5.4% MRSA nasal carriage prevalence. Compared to a composite reference standard defined as a positive test result on any one of the three assays, Hain GenoQuick MRSA demonstrated the highest sensitivity (96%) followed by direct plating on CHROMagar at (70%), with the lowest sensitivity observed with Xpert SA Complete (52%). Cepheid Xpert provided the most rapid results (< 1 h) but was the most expensive (US $45-50/test). Substantially more labor was required for the Hain GenoQuick MRSA compared to Xpert SA Complete or CHROMagar tests.
Conclusion: MRSA nasal carriage prevalence rates were low, and high diagnostic sensitivity was achieved using Hain GenoQuick MRSA. Chromogenic media had significantly lower sensitivity, but may represent a viable local option given its lower cost compared to PCR-based assays.
Keywords: Africa; Carriage; Chromogenic agar; MRSA; Methicillin-resistant Staphylococcus aureus; PCR.
Conflict of interest statement
Cepheid donated the Cepheid Xpert SA Nasal Complete assays for use in this study, and Life Science donated the Hain GenoQuick MRSA kits used in this study. All authors declare no other competing interests.
Figures
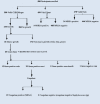
Fig. 1
Flow diagram of samples collected and results from all three testing methods
Similar articles
- Comparative evaluation of three chromogenic media combined with broth enrichment and the real-time PCR-based Xpert MRSA assay for screening of methicillin-resistant Staphylococcus aureus in nasal swabs.
Lee S, Park YJ, Park KG, Jekarl DW, Chae H, Yoo JK, Seo SW, Choi JE, Lim JH, Heo SM, Seo JH. Lee S, et al. Ann Lab Med. 2013 Jul;33(4):255-60. doi: 10.3343/alm.2013.33.4.255. Epub 2013 Jun 24. Ann Lab Med. 2013. PMID: 23826561 Free PMC article. - Multicenter evaluation of the LightCycler methicillin-resistant Staphylococcus aureus (MRSA) advanced test as a rapid method for detection of MRSA in nasal surveillance swabs.
Peterson LR, Liesenfeld O, Woods CW, Allen SD, Pombo D, Patel PA, Mehta MS, Nicholson B, Fuller D, Onderdonk A. Peterson LR, et al. J Clin Microbiol. 2010 May;48(5):1661-6. doi: 10.1128/JCM.00003-10. Epub 2010 Mar 24. J Clin Microbiol. 2010. PMID: 20335423 Free PMC article. - Methods for screening for methicillin-resistant Staphylococcus aureus carriage.
French GL. French GL. Clin Microbiol Infect. 2009 Dec;15 Suppl 7:10-6. doi: 10.1111/j.1469-0691.2009.03092.x. Clin Microbiol Infect. 2009. PMID: 19951329 Review. - Clinical effectiveness of rapid tests for methicillin resistant Staphylococcus aureus (MRSA) in hospitalized patients: a systematic review.
Polisena J, Chen S, Cimon K, McGill S, Forward K, Gardam M. Polisena J, et al. BMC Infect Dis. 2011 Dec 12;11:336. doi: 10.1186/1471-2334-11-336. BMC Infect Dis. 2011. PMID: 22151575 Free PMC article. Review.
Cited by
- Molecular diagnostics for genotypic detection of antibiotic resistance: current landscape and future directions.
Banerjee R, Patel R. Banerjee R, et al. JAC Antimicrob Resist. 2023 Feb 17;5(1):dlad018. doi: 10.1093/jacamr/dlad018. eCollection 2023 Feb. JAC Antimicrob Resist. 2023. PMID: 36816746 Free PMC article. Review. - Recent Developments in Phenotypic and Molecular Diagnostic Methods for Antimicrobial Resistance Detection in Staphylococcus aureus: A Narrative Review.
Sanchini A. Sanchini A. Diagnostics (Basel). 2022 Jan 15;12(1):208. doi: 10.3390/diagnostics12010208. Diagnostics (Basel). 2022. PMID: 35054375 Free PMC article. Review.
References
- Calfee DP, Salgado CD, Classen D, Arias KM, Podgorny K, Anderson DJ, Burstin H, Coffin SE, Dubberke ER, Fraser V, Gerding DN. Practice recommendation strategies to prevent transmission of methicillin-resistant Staphylococcus aureus in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(Suppl 1):62–80. doi: 10.1086/591061. - DOI - PubMed
- WHO. Report on the burden of endemic health care-associated infection world-wide. https://apps.who.int/iris/bitstream/handle/10665/80135/9789241501507_eng.... Accessed 16 Nov 2013.
Publication types
MeSH terms
Grants and funding
- 001/WHO_/World Health Organization/International
- K23 MH099916/MH/NIMH NIH HHS/United States
- KL2 TR001100/TR/NCATS NIH HHS/United States
- UL1 TR001102/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical