YBX-1 mediated sorting of miR-133 into hypoxia/reoxygenation-induced EPC-derived exosomes to increase fibroblast angiogenesis and MEndoT - PubMed (original) (raw)
YBX-1 mediated sorting of miR-133 into hypoxia/reoxygenation-induced EPC-derived exosomes to increase fibroblast angiogenesis and MEndoT
Fengxia Lin et al. Stem Cell Res Ther. 2019.
Abstract
Background: Myocardial fibrosis is a common pathophysiological change in cardiovascular disease, which can cause cardiac dysfunction and even sudden death. Excessively activated fibroblasts proliferate and secret excessive extracellular matrix (ECM) components, resulting in normal cardiac structural damage and cardiac fibrosis. We previously found that human endothelial progenitor cell (EPC)-derived exosomes, after hypoxia/reoxygenation (H/R) induction, could significantly increase the mesenchymal-endothelial transition (MEndoT) compared to normal culture EPC-derived exosomes. Exosomes have been shown to carry different nucleic acids, including microRNAs. However, the effects of microRNAs in EPC-derived exosomes on MEndoT and myocardial fibrosis remain unknown.
Methods: EPCs were isolated from human peripheral blood, and fibroblasts were isolated from rat hearts, then transfected with miR-133 inhibitor, si-YBX-1, and ov-YBX-1 into EPCs. After H/R induction for 48 h, isolation and characterization of exosomes derived from human EPCs were performed. Finally, fibroblasts were treated by exosome at 48 h. The expression of miR-133 was measured by qRT-PCR; YBX-1 expression was measured by qRT-PCR and western blot. Angiopoiesis was measured by tube formation assay. Endothelial markers and fibrosis markers were measured by western blot.
Results: H/R treatment promoted miR-133 expression in EPCs and EPC-derived exosomes. miR-133 could be incorporated into exosomes and transmitted to cardiac fibroblasts, increasing the angiogenesis and MEndoT of cardiac fibroblasts. miR-133 silencing in H/R-induced EPCs could inhibit miR-133 expression in EPCs and EPCs-derived exosomes. miR-133 silencing in H/R-induced EPCs could inhibit the angiogenesis and MEndoT of cardiac fibroblasts and reverse the effect of H/R treatment. Additionally, miR-133 was specially sorted into H/R-induced EPC-derived exosomes via YBX-1. YBX-1 silencing inhibited miR-133 transfer and reduced fibroblast angiogenesis and MEndoT.
Conclusion: miR-133 was specially sorted into H/R-induced EPC-derived exosomes via YBX-1 to increase fibroblast angiogenesis and MEndoT.
Keywords: Endothelial progenitor cell; Exosome; Myocardial fibrosis; Y box binding protein 1; miR-133.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Fig. 1
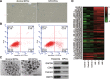
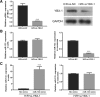
miRNA expression in H/R-induced EPC-derived exosomes. a Senescence of H/R-treated EPCs was measured by senescence β-galactosidase staining kit. Senescence cells were stained aquamarine green. b Apoptosis of H/R-treated EPCs was measured by the Annexin V-PI Apoptosis Detection Kit. c Representative electron microscopy images of exosomes secreted by EPCs. Scale bar, 200 nm. Detection of exosome-associated proteins (including CD63, TSG101, and HSP70) by western blot analysis. d Heat map showing the miRNA expression profile as measured by Affymetrix miRNA 4.0 Arrays
Fig. 2
Upregulation of miR-133, miR-218, and miR-9 expression in H/R-induced EPC-derived exosomes. Expression levels of miR-133, miR-218, and miR-9 were measured by qRT-PCR in H/R-induced and normal cultured EPC-derived exosomes. Data are shown as mean ± SD. ***P < 0.001
Fig. 3
Significant inhibition of miR-133 expression by miR-133 inhibitor transfection in H/R-induced EPCs and H/R-induced EPC-derived exosomes. miR-133 expression was measured by qRT-PCR after transfection with an NC inhibitor and miR-133 inhibitor at 48 h. Data are shown as mean ± SD. ***P < 0.001
Fig. 4
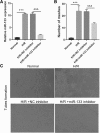
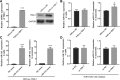
Silencing of miR-133 in H/R-induced EPCs inhibits fibroblast angiogenesis. a miR-133 expression was measured by qRT-PCR in fibroblasts treated with EPC-derived exosomes for 48 h. b The bar graph represents quantification of the number of meshes per group. c Representative image of tube formation analysis. Data are shown as mean ± SD. ***P < 0.001, &&&P < 0.001
Fig. 5
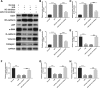
Intercellular transfer of miR-133 by H/R-induced EPC-derived exosomes inhibits fibroblast MEndoT. a Endothelial markers CD31, VE-cadherin, and vWF and fibrosis markers α-SMA, N-cadherin, vimentin, and collagen I were measured by western blotting in fibroblasts treated with normal cultured EPC-derived exosomes, H/R-induced EPC-derived exosomes, H/R+NC inhibitor-induced EPC-derived exosomes, and H/R+miR-133 inhibitor-induced EPC-derived exosomes. b–h The bar graph represents quantification of endothelial markers CD31 (b), VE-cadherin (c), and Vwf (d) and fibrosis markers α-SMA (e), N-cadherin (f), vimentin (g), and collagen I (h) expression per group. ***P < 0.001, &&&P < 0.001
Fig. 6
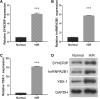
Measurements of YBX-1, SYNCRIP, and hnRNPA2B1 in EPCs by qRT-PCR (a–c) and western blotting (d) after 48-h H/R treatment
Fig. 7
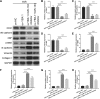
Inhibition of specifically packaged miR-133 into EPC-derived exosomes by YBP1 silencing. a qRT-PCR and western blot analysis of YBX1 expression in H/R-induced EPCs at 48 h following si-YBX1 transfection. b qRT-PCR analysis of miR-133 expression in H/R-induced EPCs and exosomes at 48 h following si-YBX1 transfection. c qRT-PCR analysis of miR-133 expression in H/R-induced EPCs and exosomes at 48 h after co-transfection with si-YBX1 and miR-133 mimic
Fig. 8
Promotion of specifically packaged miR-133 into EPC-derived exosomes by YBP1 overexpression. a qRT-PCR and western blot analysis of YBX1 expression in H/R-induced EPCs at 48 h following ov-YBX1 transfection. b qRT-PCR analysis of miR-133 expression in H/R-induced EPCs and exosomes at 48 h following ov-YBX1 transfection. c qRT-PCR analysis of miR-133 expression in H/R-induced EPCs and exosomes at 48 h after co-transfection with ov-YBX1 and miR-133 mimic. d qRT-PCR analysis of miR-133 expression in H/R-induced EPCs and exosomes at 48 h after co-transfection with ov-NC and miR-133 inhibitor or ov-YBX1 and miR-133 inhibitor
Fig. 9
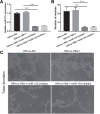
Silencing of YBX1 in H/R-induced EPCs inhibits fibroblast angiogenesis. a miR-133 expression was measured by qRT-PCR in fibroblasts treated with H/R, H/R+ov-NC, H/R+si-YBX1+miR-133 inhibitor, and H/R+ ov-YBX1+miR-133 inhibitor-induced EPC-derived exosomes for 48 h. b The bar graph represents quantification of the number of meshes per group. c Representative image of tube formation analysis. Data are shown as mean ± SD. ***P < 0.001
Fig. 10
Intercellular transfer of miR-133 by H/R-induced EPC-derived exosomes inhibits fibroblast MEndoT. a Endothelial markers CD31, VE-cadherin, and vWF and fibrosis markers α-SMA, N-cadherin, vimentin, and collagen I were measured by western blotting in fibroblasts treated with H/R, H/R+ov-NC, H/R+si-YBX1+miR-133 inhibitor, and H/R+ ov-YBX1+miR-133 inhibitor-induced EPC-derived exosomes. b–h The bar graph represents quantification of endothelial markers CD31 (b), VE-cadherin (c), and vWF (d) and fibrosis markers α-SMA (e), N-cadherin (f), vimentin (g), and collagen I (h) expression per group. ***P < 0.001
Similar articles
- Loading MiR-210 in Endothelial Progenitor Cells Derived Exosomes Boosts Their Beneficial Effects on Hypoxia/Reoxygeneation-Injured Human Endothelial Cells via Protecting Mitochondrial Function.
Ma X, Wang J, Li J, Ma C, Chen S, Lei W, Yang Y, Liu S, Bihl J, Chen C. Ma X, et al. Cell Physiol Biochem. 2018;46(2):664-675. doi: 10.1159/000488635. Epub 2018 Mar 29. Cell Physiol Biochem. 2018. PMID: 29621777 - ACE2-EPC-EXs protect ageing ECs against hypoxia/reoxygenation-induced injury through the miR-18a/Nox2/ROS pathway.
Zhang C, Wang J, Ma X, Wang W, Zhao B, Chen Y, Chen C, Bihl JC. Zhang C, et al. J Cell Mol Med. 2018 Mar;22(3):1873-1882. doi: 10.1111/jcmm.13471. Epub 2018 Jan 24. J Cell Mol Med. 2018. PMID: 29363860 Free PMC article. - Cardioprotective Roles of Endothelial Progenitor Cell-Derived Exosomes.
Zeng CY, Xu J, Liu X, Lu YQ. Zeng CY, et al. Front Cardiovasc Med. 2021 Aug 26;8:717536. doi: 10.3389/fcvm.2021.717536. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34513956 Free PMC article. Review. - Exercise improves cardiac fibrosis by stimulating the release of endothelial progenitor cell-derived exosomes and upregulating miR-126 expression.
Fu G, Wang Z, Hu S. Fu G, et al. Front Cardiovasc Med. 2024 May 9;11:1323329. doi: 10.3389/fcvm.2024.1323329. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 38798919 Free PMC article. Review.
Cited by
- Fabrication of Tβ4-Exosome-releasing artificial stem cells for myocardial infarction therapy by improving coronary collateralization.
Chen P, Ning X, Li W, Pan Y, Wang L, Li H, Fan X, Zhang J, Luo T, Wu Y, Ou C, Chen M. Chen P, et al. Bioact Mater. 2022 Jan 29;14:416-429. doi: 10.1016/j.bioactmat.2022.01.029. eCollection 2022 Aug. Bioact Mater. 2022. PMID: 35386821 Free PMC article. - Exosomal MiRNAs in Osteosarcoma: Biogenesis and Biological Functions.
Tang J, He J, Feng C, Tu C. Tang J, et al. Front Pharmacol. 2022 May 3;13:902049. doi: 10.3389/fphar.2022.902049. eCollection 2022. Front Pharmacol. 2022. PMID: 35592419 Free PMC article. Review. - Mechanism of cargo sorting into small extracellular vesicles.
Chen Y, Zhao Y, Yin Y, Jia X, Mao L. Chen Y, et al. Bioengineered. 2021 Dec;12(1):8186-8201. doi: 10.1080/21655979.2021.1977767. Bioengineered. 2021. PMID: 34661500 Free PMC article. - Exosomes in the hypoxic TME: from release, uptake and biofunctions to clinical applications.
He G, Peng X, Wei S, Yang S, Li X, Huang M, Tang S, Jin H, Liu J, Zhang S, Zheng H, Fan Q, Liu J, Yang L, Li H. He G, et al. Mol Cancer. 2022 Jan 17;21(1):19. doi: 10.1186/s12943-021-01440-5. Mol Cancer. 2022. PMID: 35039054 Free PMC article. Review. - Exosomes in Mastitis-Research Status, Opportunities, and Challenges.
Ji ZH, Ren WZ, Wu HY, Zhang JB, Yuan B. Ji ZH, et al. Animals (Basel). 2022 Oct 21;12(20):2881. doi: 10.3390/ani12202881. Animals (Basel). 2022. PMID: 36290266 Free PMC article. Review.
References
- Bittencourt MI, Cader SA, Araujo DV, Salles ALF, Albuquerque FN, Spineti PPM, Albuquerque DC, Mourilhe-Rocha R. Role of myocardial fibrosis in hypertrophic cardiomyopathy: a systematic review and updated meta-analysis of risk markers for sudden death. Arq Bras Cardiol. 2019;112:281–289. - PMC - PubMed
- Ke X, Yang D, Liang J, Wang X, Wu S, Wang X, Hu C. Human endothelial progenitor cell-derived exosomes increase proliferation and angiogenesis in cardiac fibroblasts by promoting the mesenchymal-endothelial transition and reducing high mobility group box 1 protein B1 expression. DNA Cell Biol. 2017;36:1018–1028. doi: 10.1089/dna.2017.3836. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials