Neutral Lipopolyplexes for In Vivo Delivery of Conventional and Replicative RNA Vaccine - PubMed (original) (raw)
Neutral Lipopolyplexes for In Vivo Delivery of Conventional and Replicative RNA Vaccine
Federico Perche et al. Mol Ther Nucleic Acids. 2019.
Abstract
Nucleic acid vaccination relies on injecting DNA or RNA coding antigen(s) to induce a protective immune response. RNA vaccination is being increasingly used in preclinical and clinical studies. However, few delivery systems have been reported for in vivo delivery of RNA of different sizes. Using a tripartite formulation with RNA, cationic polymer, and anionic liposomes, we were able to encapsulate RNA into neutral lipopolyplexes (LPPs). LPPs were stable in vitro and successfully delivered conventional RNA and replicative RNA to dendritic cells in cellulo. Their injection led to reporter gene expression in mice. Finally, administration of LPP-Replicon RNA (RepRNA) led to an adaptive immune response against the antigen coded by the RepRNA. Accordingly, LPPs may represent a universal formulation for RNA delivery.
Keywords: mRNA delivery; self-amplifying RNA; splenic dendritic cells; targeting.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
Figure 1
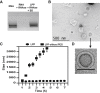
Formation of LPPs
Figure 2
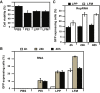
LPP Morphology and Stability (A) RNase protection assay: lane 1, RNA; lane 2, RNA + RNase; lane 3, LPPs treated with RNase and dissociated with sulfated dextran (SD). (B) Morphology of LPPs by TEM at low magnification. Scale bar represents 500 nm. (C) Measurement of LPP diameters by DLS after incubation in media 10% FBS at 37°C. (D) Morphology of LPPs by TEM. Scale bar represents 100 nm.
Figure 3
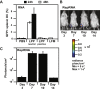
LPP Activity in Murine Dendritic Cells In Cellulo (A) DC transfection efficiency of PEI, LPP, and LFM formulations made with 2 μg GFP mRNA. (B) Cell viability of DCs 24 h after transfection with PEI 25K polyplexes, LPPs, or lipofectamine messenger max (LFM) complexes. (C) DC transfection efficiency of LPPs or LFM made with 2 μg RepRNA.
Figure 4
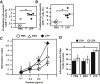
In Vivo RNA Expression after LPP-RNA Administration (A) Percentages of murine splenic DC transfection after intravenous injection of LPP-RNA complexes (20 μg in 250 μL). (B) Bioluminescence imaging of BALB/c mice at various days following intramuscular injection of LPP-RepRNA complexes (5 μg in 50 μL); mean luminescence values are also shown in photons/seconds/cm2.
Figure 5
Immunogenicity of LPP-RepRNA (A and B) Mice were vaccinated by intramuscular injection of LPP-RepRNA. We analyzed the induction of HA-specific T cells in the lymph nodes, both CD4 (A) and CD8 (B). Stimulation indexes of splenocytes from vaccinated mice after re-stimulation with HA peptide (C) and counts of IFN-γ secreting splenic T cells. **p < 0.01 compared with RepRNA.
Similar articles
- Self-Replicating RNA Vaccine Delivery to Dendritic Cells.
Démoulins T, Englezou PC, Milona P, Ruggli N, Tirelli N, Pichon C, Sapet C, Ebensen T, Guzmán CA, McCullough KC. Démoulins T, et al. Methods Mol Biol. 2017;1499:37-75. doi: 10.1007/978-1-4939-6481-9_3. Methods Mol Biol. 2017. PMID: 27987142 Review. - Self-Amplifying Replicon RNA Delivery to Dendritic Cells by Cationic Lipids.
Englezou PC, Sapet C, Démoulins T, Milona P, Ebensen T, Schulze K, Guzman CA, Poulhes F, Zelphati O, Ruggli N, McCullough KC. Englezou PC, et al. Mol Ther Nucleic Acids. 2018 Sep 7;12:118-134. doi: 10.1016/j.omtn.2018.04.019. Epub 2018 May 4. Mol Ther Nucleic Acids. 2018. PMID: 30195751 Free PMC article. - Polyethylenimine-based polyplex delivery of self-replicating RNA vaccines.
Démoulins T, Milona P, Englezou PC, Ebensen T, Schulze K, Suter R, Pichon C, Midoux P, Guzmán CA, Ruggli N, McCullough KC. Démoulins T, et al. Nanomedicine. 2016 Apr;12(3):711-722. doi: 10.1016/j.nano.2015.11.001. Epub 2015 Dec 1. Nanomedicine. 2016. PMID: 26592962 - Self-replicating Replicon-RNA Delivery to Dendritic Cells by Chitosan-nanoparticles for Translation In Vitro and In Vivo.
McCullough KC, Bassi I, Milona P, Suter R, Thomann-Harwood L, Englezou P, Démoulins T, Ruggli N. McCullough KC, et al. Mol Ther Nucleic Acids. 2014 Jul 8;3(7):e173. doi: 10.1038/mtna.2014.24. Mol Ther Nucleic Acids. 2014. PMID: 25004099 Free PMC article. - Self-Amplifying Replicon RNA Vaccine Delivery to Dendritic Cells by Synthetic Nanoparticles.
McCullough KC, Milona P, Thomann-Harwood L, Démoulins T, Englezou P, Suter R, Ruggli N. McCullough KC, et al. Vaccines (Basel). 2014 Oct 16;2(4):735-54. doi: 10.3390/vaccines2040735. Vaccines (Basel). 2014. PMID: 26344889 Free PMC article. Review.
Cited by
- Designing Polyelectrolyte Microneedles Based on Borylated Poly(β-aminoester) Polymers To Enhance Transdermal pH-Controlled Delivery of Nucleic Acids.
González-Sáenz P, Cosialls R, Texidó R, Dols-Pérez A, Cuenca AB, Borrós S, Fornaguera C. González-Sáenz P, et al. ACS Appl Polym Mater. 2024 Jul 24;6(15):8842-8855. doi: 10.1021/acsapm.4c00969. eCollection 2024 Aug 9. ACS Appl Polym Mater. 2024. PMID: 39144279 Free PMC article. - Oligonucleotide therapies for nonalcoholic steatohepatitis.
Li S, Xiong F, Zhang S, Liu J, Gao G, Xie J, Wang Y. Li S, et al. Mol Ther Nucleic Acids. 2024 Mar 30;35(2):102184. doi: 10.1016/j.omtn.2024.102184. eCollection 2024 Jun 11. Mol Ther Nucleic Acids. 2024. PMID: 38665220 Free PMC article. Review. - Advances in saRNA Vaccine Research against Emerging/Re-Emerging Viruses.
Liu Y, Li Y, Hu Q. Liu Y, et al. Vaccines (Basel). 2023 Jun 24;11(7):1142. doi: 10.3390/vaccines11071142. Vaccines (Basel). 2023. PMID: 37514957 Free PMC article. Review. - Transitional Insight into the RNA-Based Oligonucleotides in Cancer Treatment.
Tabasi H, Mollazadeh S, Fazeli E, Abnus K, Taghdisi SM, Ramezani M, Alibolandi M. Tabasi H, et al. Appl Biochem Biotechnol. 2024 Mar;196(3):1685-1711. doi: 10.1007/s12010-023-04597-5. Epub 2023 Jul 4. Appl Biochem Biotechnol. 2024. PMID: 37402038 Review. - Nanomaterials for mRNA-based therapeutics: Challenges and opportunities.
Li DF, Liu QS, Yang MF, Xu HM, Zhu MZ, Zhang Y, Xu J, Tian CM, Yao J, Wang LS, Liang YJ. Li DF, et al. Bioeng Transl Med. 2023 Jan 29;8(3):e10492. doi: 10.1002/btm2.10492. eCollection 2023 May. Bioeng Transl Med. 2023. PMID: 37206219 Free PMC article. Review.
References
- Sahin U., Karikó K., Türeci Ö. mRNA-based therapeutics--developing a new class of drugs. Nat. Rev. Drug Discov. 2014;13:759–780. - PubMed
- Perche F., Uchida S., Akiba H., Lin C.-Y., Ikegami M., Dirisala A., Nakashima T., Itaka K., Tsumoto K., Kataoka K. Improved brain expression of anti-amyloid β scfv by complexation of mRNA including a secretion sequence with PEG-based block catiomer. Curr. Alzheimer Res. 2017;14:295–302. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials