Evidence that the nadA motif is a bacterial riboswitch for the ubiquitous enzyme cofactor NAD - PubMed (original) (raw)
Evidence that the nadA motif is a bacterial riboswitch for the ubiquitous enzyme cofactor NAD
Sarah N Malkowski et al. RNA. 2019 Dec.
Abstract
The nadA motif is a riboswitch candidate present in various Acidobacteria species that was previously identified by bioinformatic analysis of bacterial DNA data sets. More than 100 unique representatives have been identified exclusively upstream of nadA genes, which code for an enzyme in the biosynthetic pathway of the ubiquitous coenzyme NAD+ The architecture of nadA motif RNAs suggests they use structurally similar tandem ligand-binding aptamer domains to control translation initiation. Biochemical analyses reveal that the first domain selectively binds ligands carrying an adenosine 5'-diphosphate (5' ADP) moiety, including NAD+ and its reduced form, NADH. Genetic analyses indicate that a tandem nadA motif RNA suppresses gene expression when NAD+ is abundant, and that both aptamer domains are required for maximal gene regulation. However, we have not observed selective binding of the nicotinamide moiety of NAD+ or binding by the second putative aptamer in vitro, despite sequence and structural similarities between the tandem domains.
Keywords: adenosine-5'-diphosphate; aptamer; gene control; nicotinamide adenine dinucleotide; noncoding RNA.
© 2019 Malkowski et al.; Published by Cold Spring Harbor Laboratory Press for the RNA Society.
Figures
FIGURE 1.
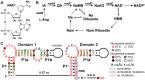
Tandem nadA motif domains are found upstream of genes in the biosynthetic pathway for NAD+. (A) The chemical structure of NAD+. (B) Biosynthetic pathway of NAD+ and related molecules (Stancek et al. 2005; Zhou et al. 2011). Note that all nadA motif representatives are found exclusively upstream of nadA genes. (
l
-Asp)
l
-aspartate, (IA) iminoaspartate, (QA) quinolinic acid, (NaMN) nicotinic acid mononucleotide, (NaAD) nicotinic acid adenine dinucleotide, (NMN) nicotinamide mononucleotide, (NADP+) nicotinamide adenosine dinucleotide phosphate, (Na) nicotinic acid, (Nam) nicotinamide. Riboside derivatives carry a ribose moiety linked by an N-glycosidic linkage to the nicotinic or nicotinamide ring nitrogen atom. (C) Consensus sequence and secondary structure model for 101 unique examples of tandem nadA motif RNAs. The putative ribosome binding site (RBS) is followed by a predicted start codon for the adjacent nadA open reading frame (ORF).
FIGURE 2.
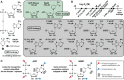
Domain 1 of nadA motif RNAs bind NAD+ and NADH in vitro. (A) Sequence and secondary structure model of the 61 nadA RNA construct from A. ailaaui. Lowercase nucleotides on the 5′ terminus designate G residues added to facilitate production by T7 RNA polymerase. Sites of spontaneous RNA cleavage revealed by an in-line probing assay depicted in B are circled. Red circles identify nucleotide linkages that undergo suppression of spontaneous cleavage in the presence of added ligand, whereas yellow circles identify linkages that cleave at the same rate regardless of ligand addition. Numbered regions were used to quantify ligand binding as presented in C. (B) Denaturing (8 M urea) polyacrylamide gel electrophoresis (PAGE) analysis of in-line probing reactions conducted with 5′ 32P-labeled 61 nadA RNA in the absence (−) or presence of NADH, ranging from 100 to 4.64 mM. NR, T1, and −OH indicate no reaction, partial digestion with T1 ribonuclease (cleaves after every G), and partial digestion under alkaline conditions (cleaves after every nucleotide), respectively. Precursor RNA (Pre) and certain G residues are annotated, and regions of modulation (vertical bars) are numbered as designated in A. (C) Plot of the fraction of RNA bound to ligand versus the logarithm of the NADH concentration as determined by quantification of the PAGE data in B. See the Materials and Methods section for details. (D) Sequence and secondary structure model of the 75 nadA RNA construct from E. aggregans, containing only domain 1. Mutations M1–M4 alter highly conserved (>97%) nucleotides (boxed and annotated with the mutant nucleotide identity) to assess their requirement for ligand binding activity. (E) PAGE analysis of in-line probing reactions of 5′ 32P-labeled 75 nadA RNA in the absence (−) or presence of NAD+ ranging from 100 to 4.64 mM. Annotations are as describe for B. (F) Plot of the fraction of RNA bound to ligand versus the logarithm of the NAD+ concentration as determined by quantification of the PAGE data in E.
FIGURE 3.
SAR data for ligand binding by domain 1 of a nadA RNA construct. (A) Chemical structures of various ligand candidates examined for binding of the 75 nadA RNA construct from E. aggregans (Fig. 2D) by using in-line probing assays. All compounds were initially screened for binding at 1 mM, and a dissociation constant was obtained for compounds that induced structural modulation. (B) The plot of the _K_D values for compounds recognized by domain 1, as determined by quantification of PAGE analyses of in-line probing reactions (data not shown). (C) (Left) The chemical structure of 5′ ADP and the proposed sites of molecular recognition by the RNA aptamer formed by domain 1. Arrows represent proposed sites for hydrogen bond acceptor (red) and donor (blue) function. (Right) The chemical structure of phosphorylated NMN (NMN*) depicted with the recognition sites used by domain 1 to bind ADP.
FIGURE 4.
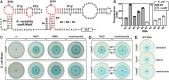
Gene regulation by a tandem nadA motif RNA. (A) Sequence and secondary structure of the wild-type (WT) nadA RNA aptamer from K. versatilis. Red nucleotides are >97% conserved as depicted in the nadA motif consensus model (Fig. 1C). Mutant constructs (M1–M4) carry nucleotide changes at the indicated positions. (B) The plot of the relative β-galactosidase expression of an E. coli strain carrying a WT or mutant nadA riboswitch-lacZ reporter fusion construct in WT cells or a genomic disruption to the nadR gene. Cells were grown in LB for 6 h before quantification. (C) M9 minimal media agar plates containing 100 µg mL−1 X-gal were streaked with E. coli strains carrying a WT riboswitch reporter fusion construct and with genomic disruptions to nadA or nadC genes. Plates contain a filter disk supplemented with 5 μL dH2O (−) or with 5 μL of a 10 mM solution containing a nicotinamide derivative. (D) M9 minimal media agar plates containing 100 µg mL−1 X-gal were streaked with E. coli strains with genomic disruptions to nadA or nadC genes and carrying a WT or M1 riboswitch reporter fusion construct. Plates contain a filter disk supplemented with the nicotinamide derivative noted. (E) Agar diffusion assays using the WT riboswitch reporter fusion construct hosted by various additional strains of E. coli that are auxotrophic for compounds unrelated to NAD+. Filter disks were supplemented with 5 µL of a 10 mM solution containing the amino acid noted. Additional details are as described for C.
Similar articles
- A second riboswitch class for the enzyme cofactor NAD.
Panchapakesan SSS, Corey L, Malkowski SN, Higgs G, Breaker RR. Panchapakesan SSS, et al. RNA. 2021 Jan;27(1):99-105. doi: 10.1261/rna.077891.120. Epub 2020 Oct 21. RNA. 2021. PMID: 33087526 Free PMC article. - Structural distinctions between NAD+ riboswitch domains 1 and 2 determine differential folding and ligand binding.
Chen H, Egger M, Xu X, Flemmich L, Krasheninina O, Sun A, Micura R, Ren A. Chen H, et al. Nucleic Acids Res. 2020 Dec 2;48(21):12394-12406. doi: 10.1093/nar/gkaa1029. Nucleic Acids Res. 2020. PMID: 33170270 Free PMC article. - Structure and ligand binding of the ADP-binding domain of the NAD+ riboswitch.
Huang L, Wang J, Lilley DMJ. Huang L, et al. RNA. 2020 Jul;26(7):878-887. doi: 10.1261/rna.074898.120. Epub 2020 Apr 15. RNA. 2020. PMID: 32295864 Free PMC article. - [The adenine riboswitch: a new gene regulation mechanism].
Lemay JF, Lafontaine DA. Lemay JF, et al. Med Sci (Paris). 2006 Dec;22(12):1053-9. doi: 10.1051/medsci/200622121053. Med Sci (Paris). 2006. PMID: 17156726 Review. French. - Former orphan riboswitches reveal unexplored areas of bacterial metabolism, signaling, and gene control processes.
Sherlock ME, Breaker RR. Sherlock ME, et al. RNA. 2020 Jun;26(6):675-693. doi: 10.1261/rna.074997.120. Epub 2020 Mar 12. RNA. 2020. PMID: 32165489 Free PMC article. Review.
Cited by
- Discovering riboswitches: the past and the future.
Kavita K, Breaker RR. Kavita K, et al. Trends Biochem Sci. 2023 Feb;48(2):119-141. doi: 10.1016/j.tibs.2022.08.009. Epub 2022 Sep 20. Trends Biochem Sci. 2023. PMID: 36150954 Free PMC article. Review. - Biochemical Validation of a Fourth Guanidine Riboswitch Class in Bacteria.
Salvail H, Balaji A, Yu D, Roth A, Breaker RR. Salvail H, et al. Biochemistry. 2020 Dec 15;59(49):4654-4662. doi: 10.1021/acs.biochem.0c00793. Epub 2020 Nov 25. Biochemistry. 2020. PMID: 33236895 Free PMC article. - Crystal structures of the NAD+-II riboswitch reveal two distinct ligand-binding pockets.
Peng X, Liao W, Lin X, Lilley DMJ, Huang L. Peng X, et al. Nucleic Acids Res. 2023 Apr 11;51(6):2904-2914. doi: 10.1093/nar/gkad102. Nucleic Acids Res. 2023. PMID: 36840714 Free PMC article. - Variants of the guanine riboswitch class exhibit altered ligand specificities for xanthine, guanine, or 2'-deoxyguanosine.
Hamal Dhakal S, Panchapakesan SSS, Slattery P, Roth A, Breaker RR. Hamal Dhakal S, et al. Proc Natl Acad Sci U S A. 2022 May 31;119(22):e2120246119. doi: 10.1073/pnas.2120246119. Epub 2022 May 27. Proc Natl Acad Sci U S A. 2022. PMID: 35622895 Free PMC article. - Riboswitches, from cognition to transformation.
Xu J, Hou J, Ding M, Wang Z, Chen T. Xu J, et al. Synth Syst Biotechnol. 2023 Jun 3;8(3):357-370. doi: 10.1016/j.synbio.2023.05.008. eCollection 2023 Sep. Synth Syst Biotechnol. 2023. PMID: 37325181 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources