Small Vessels Are a Big Problem in Neurodegeneration and Neuroprotection - PubMed (original) (raw)
Review
Small Vessels Are a Big Problem in Neurodegeneration and Neuroprotection
Şefik Evren Erdener et al. Front Neurol. 2019.
Abstract
The cerebral microcirculation holds a critical position to match the high metabolic demand by neuronal activity. Functionally, microcirculation is virtually inseparable from other nervous system cells under both physiological and pathological conditions. For successful bench-to-bedside translation of neuroprotection research, the role of microcirculation in acute and chronic neurodegenerative disorders appears to be under-recognized, which may have contributed to clinical trial failures with some neuroprotectants. Increasing data over the last decade suggest that microcirculatory impairments such as endothelial or pericyte dysfunction, morphological irregularities in capillaries or frequent dynamic stalls in blood cell flux resulting in excessive heterogeneity in capillary transit may significantly compromise tissue oxygen availability. We now know that ischemia-induced persistent abnormalities in capillary flow negatively impact restoration of reperfusion after recanalization of occluded cerebral arteries. Similarly, microcirculatory impairments can accompany or even precede neural loss in animal models of several neurodegenerative disorders including Alzheimer's disease. Macrovessels are relatively easy to evaluate with radiological or experimental imaging methods but they cannot faithfully reflect the downstream microcirculatory disturbances, which may be quite heterogeneous across the tissue at microscopic scale and/or happen fast and transiently. The complexity and size of the elements of microcirculation, therefore, require utilization of cutting-edge imaging techniques with high spatiotemporal resolution as well as multidisciplinary team effort to disclose microvascular-neurodegenerative connection and to test treatment approaches to advance the field. Developments in two photon microscopy, ultrafast ultrasound, and optical coherence tomography provide valuable experimental tools to reveal those microscopic events with high resolution. Here, we review the up-to-date advances in understanding of the primary microcirculatory abnormalities that can result in neurodegenerative processes and the combined neurovascular protection approaches that can prevent acute as well as chronic neurodegeneration.
Keywords: capillary; flow heterogeneity; microcirculation; neurodegeneration; neuroprotection; oxygen extraction; pericytes.
Figures
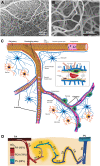
Figure 1
Morphological features of cerebral microcirculation. (A,B) Dense external coverage of the surface by pial arteries and veins which are interconnected via anastomoses and collateral vessels then gives rise to a complex meshwork of capillaries, composed of segments about 60–70 μm in length. (C) Penetrating arteries originating from pial vasculature dive into the cortical tissue without forming any further anastomoses. Immediately surrounded by the cerebrospinal fluid-filled spaces called perivascular spaces or Virchow-Robin spaces along their course, penetrating arteries then give rise an extensive tree of small vessels as they branch into arterioles (30–100 μm diameter), precapillary arterioles (10–30 μm), and capillaries (<10 μm), respectively. As arterioles turn into capillaries, the perivascular space disappears, making the capillary wall adjacent to the parenchyma. (D) Capillaries branch out for 5–6 times on average (2) as they release oxygen into the tissue, until they converge on postcapillary venules that drain into ascending veins. (A,B reproduced by permission from: PNAS, Meyer et al. (3) ©2008 National Academy of Sciences. (D) reprinted by permission from: Nature Communications, Sakadžić et al. (2) ©2014 Springer Nature).
Figure 2
Irregularity and heterogeneity in capillary flow (A) OCT angiogram time-series identify capillary segments with stalling red blood cells. Individual segments (arrowheads) with temporary interruptions of RBC flux simply lose OCT angiogram signal. Hollow arrowheads indicate a stalled capillary segment (10). (B) Two photon microscopy time series with fluorescent labeled plasma can identify flowing and stalled capillaries based on the motion of unlabeled cells as seen in black (11). (C) A single time-point two-photon angiogram of a set of capillary branches at ~100 μm below cortical surface shows heterogeneous distribution of RBC flux. Segments with higher flow have thinner and denser RBC-bands whereas slower flow is indicated by thicker and more scarce bands. One segment (arrow) has no RBCs flowing but is still filled with fluorescent labeled plasma. Scalebar: 20 μm) (D) Matrix plot of individual stall events in a region of interest, acquired during a functional stimulation experiment, with a frame period of 9 s. Every black points denotes a stall in a particular capillary. Green shades indicate whisker stimulation. (E) The frequency of capillary stalls is dynamically modulated during functional stimulation; stall prevalence was significantly lower during functional hyperemia. *Statistical significance (p < 0.05). (A,D,E reproduced by permission from: JCBFM, Erdener et al. (10); (B) reprinted by permission from: Nature Neuroscience, Cruz Hernandez et al. (11), ©2019 Springer Nature).
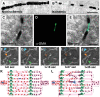
Figure 3
Capillary dysfunction in ischemic stroke and flow-limiting conditions. (A,B) Differential interference contrast (DIC) microscopy images illustrate frequent interruptions in the erythrocyte column in an ischemic capillary contrary to a continuous row of erythrocytes flowing through a non-ischemic capillary. Scale bar: 20 μm. (C–E) The constricted segments colocalized with α-smooth muscle actin (α-SMA) immunoreactive pericytes. Scale bar: 10 μm. (F–J) Very high frequency of dynamic RBC flow stalls in ischemic penumbra shown with OCT angiogram time-series (Manuscript* in preparation). (K) Ideally, capillary flow should be homogeneous across a capillary bed to optimize oxygen extraction. Arrows indicate direction of cell motion. (L) Pericyte contractions, and increased plugging of leukocytes and red blood cells as a result of ischemia-induced capillary dysfunction, introduce severe heterogeneity into the microcirculation, resulting in redistributions of flow, and pathological shunting. This can profoundly reduce the oxygen delivery into the tissue, even if the total plasma-perfused capillary count and absolute arterial input is the same. Green arrows indicate constricted pericytes, red arrowheads indicate stagnant red blood cells, blue arrow indicates a plugged leukocyte. Deoxygenated RBCs are darker and bluish in color. [A–E reproduced by permission from: Nature Medicine, Yemisci et al. (28)]. (*Manuscript by authors: Erdener SE, Tang J, Kilic K, Postnov D, Giblin JT, Kura S, Chen A, Vayisoglu T, Sakadzic S, Schaffer CB and Boas DA).
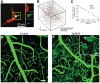
Figure 4
Microcirculatory changes in experimental models of Alzheimer's disease. (A) There is increased fraction of stalled capillaries in double-transgenic mice overexpressing amyloid-beta (APP/SW1) due to increased plugging by leukocytes (Green: rhodamine-G labeled leukocyte, red: Texas-red labeled plasma). (B) Vascular network tracings show distribution of stalled capillaries. (C) Although the fraction of stalled capillaries may seem small, computer simulations on capillary networks show a prominent decrease in overall cerebral blood flow with gradual introduction of stalls. (D,E) In another model, 15 month-old mice overexpressing pathological form of hyperphosphorylated tau (Tg4510) show abnormal capillary morphology, number and density (spiral shapes as shown with asterisks(*)). Scale bar 50 μm (inset: 20 μm) There is increased number of stagnant of leukocytes also in these capillaries (not shown here). (A–C reprinted by permission from: Nature Neuroscience, Cruz Hernandez (11) ©2019 Springer Nature; (D,E) reproduced from Bennett et al. (33), by rights granted under a Creative Commons BY-NC-ND license).
Similar articles
- Pericytes in Ischemic Stroke.
Dalkara T, Alarcon-Martinez L, Yemisci M. Dalkara T, et al. Adv Exp Med Biol. 2019;1147:189-213. doi: 10.1007/978-3-030-16908-4_9. Adv Exp Med Biol. 2019. PMID: 31147879 - Cerebral microvascular pericytes and neurogliovascular signaling in health and disease.
Dalkara T, Alarcon-Martinez L. Dalkara T, et al. Brain Res. 2015 Oct 14;1623:3-17. doi: 10.1016/j.brainres.2015.03.047. Epub 2015 Apr 8. Brain Res. 2015. PMID: 25862573 Review. - In Vivo Observations of Rapid Scattered Light Changes Associated with Neurophysiological Activity.
Rector DM, Yao X, Harper RM, George JS. Rector DM, et al. In: Frostig RD, editor. In Vivo Optical Imaging of Brain Function. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 5. In: Frostig RD, editor. In Vivo Optical Imaging of Brain Function. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; 2009. Chapter 5. PMID: 26844322 Free Books & Documents. Review. - Brain microvascular pericytes in health and disease.
Dalkara T, Gursoy-Ozdemir Y, Yemisci M. Dalkara T, et al. Acta Neuropathol. 2011 Jul;122(1):1-9. doi: 10.1007/s00401-011-0847-6. Epub 2011 Jun 9. Acta Neuropathol. 2011. PMID: 21656168 Review. - Endothelial C-Type Natriuretic Peptide Acts on Pericytes to Regulate Microcirculatory Flow and Blood Pressure.
Špiranec K, Chen W, Werner F, Nikolaev VO, Naruke T, Koch F, Werner A, Eder-Negrin P, Diéguez-Hurtado R, Adams RH, Baba HA, Schmidt H, Schuh K, Skryabin BV, Movahedi K, Schweda F, Kuhn M. Špiranec K, et al. Circulation. 2018 Jul 31;138(5):494-508. doi: 10.1161/CIRCULATIONAHA.117.033383. Circulation. 2018. PMID: 29626067
Cited by
- Red Blood Cell-Mediated S-Nitrosohemoglobin-Dependent Vasodilation: Lessons Learned from a β-Globin Cys93 Knock-In Mouse.
Premont RT, Reynolds JD, Zhang R, Stamler JS. Premont RT, et al. Antioxid Redox Signal. 2021 Apr 20;34(12):936-961. doi: 10.1089/ars.2020.8153. Epub 2020 Jul 23. Antioxid Redox Signal. 2021. PMID: 32597195 Free PMC article. Review. - Alterations of the Whole Cerebral Blood Flow in Patients With Different Total Cerebral Small Vessel Disease Burden.
Yu C, Lu W, Qiu J, Wang F, Li J, Wang L. Yu C, et al. Front Aging Neurosci. 2020 Jun 23;12:175. doi: 10.3389/fnagi.2020.00175. eCollection 2020. Front Aging Neurosci. 2020. PMID: 32655393 Free PMC article. - Full-field amplitude speckle decorrelation angiography.
Mansutti G, Villiger M, Bouma BE, Uribe-Patarroyo N. Mansutti G, et al. Biomed Opt Express. 2024 Sep 6;15(10):5756-5772. doi: 10.1364/BOE.530993. eCollection 2024 Oct 1. Biomed Opt Express. 2024. PMID: 39421771 Free PMC article. - Origins of 1/f-like tissue oxygenation fluctuations in the murine cortex.
Zhang Q, Gheres KW, Drew PJ. Zhang Q, et al. PLoS Biol. 2021 Jul 15;19(7):e3001298. doi: 10.1371/journal.pbio.3001298. eCollection 2021 Jul. PLoS Biol. 2021. PMID: 34264930 Free PMC article. - Deep learning toolbox for automated enhancement, segmentation, and graphing of cortical optical coherence tomography microangiograms.
Stefan S, Lee J. Stefan S, et al. Biomed Opt Express. 2020 Nov 24;11(12):7325-7342. doi: 10.1364/BOE.405763. eCollection 2020 Dec 1. Biomed Opt Express. 2020. PMID: 33409000 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources