Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer's disease markers in subjects with mild cognitive impairment - PubMed (original) (raw)
Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer's disease markers in subjects with mild cognitive impairment
Ravinder Nagpal et al. EBioMedicine. 2019 Sep.
Abstract
Background: Alzheimer's disease (AD) prevalence is increasing, but its etiology remains elusive. Gut microbes can contribute to AD pathology and may help identifying novel markers and therapies against AD. Herein, we examine how the gut microbiome differs in older adults with mild cognitive impairment compared to cognitively normal counterparts, and whether and how a modified Mediterranean-ketogenic diet (MMKD) alters the gut microbiome signature in association with cerebrospinal fluid (CSF) AD biomarkers.
Methods: A randomized, double-blind, cross-over, single-center pilot study of MMKD versus American Heart Association Diet (AHAD) intervention is performed on 17 subjects (age: 64.6 ± 6.4 yr), of which 11 have mild cognitive impairment, while 6 are cognitively normal. Subjects undergo MMKD and AHAD intervention for 6-weeks separated by 6-weeks washout periods. Gut microbiome, fecal short-chain fatty acids (SCFAs), and markers of AD in CSF including amyloid β (Aβ)-40 and Aß-42, total tau, and phosphorylated tau-181 (tau-p181) are measured at before and after diet interventions.
Findings: At baseline, subjects with normal vs. impaired cognition show no notable difference in microbiome diversity but several unique microbial signatures are detected in subjects with mild cognitive impairment. Proteobacteria correlate positively with Aβ-42: Aβ-40 while fecal propionate and butyrate correlates negatively with Aβ-42 in subjects with mild cognitive impairment. Several bacteria are differently affected by the two diets with distinct patterns between cognitively normal and impaired subjects. Notably, the abundance of Enterobacteriaceae, Akkermansia, Slackia, Christensenellaceae and Erysipelotriaceae increases while that of Bifidobacterium and Lachnobacterium reduces on MMKD, while AHAD increases Mollicutes. MMKD slightly reduces fecal lactate and acetate while increasing propionate and butyrate. Conversely, AHAD increases acetate and propionate while reducing butyrate.
Interpretation: The data suggest that specific gut microbial signatures may depict the mild cognitive impairment and that the MMKD can modulate the gut microbiome and metabolites in association with improved AD biomarkers in CSF.
Keywords: Alzheimer; Dementia; Diet; High fat; Ketogenic; Microbiota; Nutrition; Short-chain fatty acids.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
Fig. 1
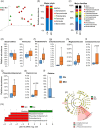
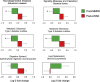
Differences in the gut microbiome between subjects clinically diagnosed with mild cognitive impairment (MCI) versus cognitively normal (CN) counterparts. (a) Microbiome beta-diversity in terms of weighted unifrac distance, and (b-c) gut microbiome composition at the level of major phyla (b) and families (c) in CN (n = 6) and MCI subjects (n = 11). (d-l) Differences in the relative abundance of bacterial taxa in the gut microbiome of CN versus MCI subjects (*p < .05); (m-n): Linear discriminant analysis (LDA) effect size (Lefse) plot and cladogram representing the unique bacterial signatures identified in CN and MCI subjects.
Fig. 2
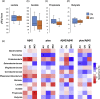
Gut bacterial taxa and fecal organic acids correlate with cerebral spinal fluid (CSF) biomarkers of Alzheimer's disease (AD) differently in subjects clinically diagnosed with mild cognitive impairment (MCI) versus cognitively normal (CN) counterparts. (a-d) Fecal concentration of organic acids including lactate and short-chain fatty acids in CN (n = 6) versus MCI subjects (n = 11). (e) Correlation (Spearman R; *p < .05) of gut bacterial taxa and fecal organic acids with CSF biomarkers of AD in CN versus MCI subjects.
Fig. 3
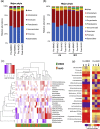
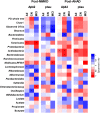
Mediterranean-style ketogenic diet (MMKD) and American Heart Association Diet (AHAD) differently influence gut microbiome in subjects clinically diagnosed with mild cognitive impairment (MCI) versus cognitively normal (CN) counterparts. (a-b) Phyla-level gut microbiome composition at baseline and endpoint of 6-weeks MMKD and AHAD intervention in all participants (n = 17) (a) and separately in CN (n = 6) versus MCI subjects (n = 11) (b). (c) Hierarchical clustering heat-map of gut bacterial taxa that showed >1.0 or < −1.0 Log2-fold increase or decrease in relative abundance during MMKD or AHAD intervention. (d) Heat-map summarizing the differential patterns of diet-induced alterations (mean Log2-fold change in relative abundance) in gut bacterial taxa that showed >1.0 or < −1.0 Log2-fold increase or decrease in relative abundance during MMKD or AHAD intervention in CN and MCI subjects.
Fig. 4
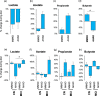
Mediterranean-style ketogenic diet (MMKD) and American Heart Association Diet (AHAD) induce specific changes in the gut microbiome of subjects clinically diagnosed with mild cognitive impairment (MCI) versus cognitively normal (CN) counterparts. Mean Log2-fold change in the relative abundance of major phyla (a), families (b) and genera (c) in CN (n = 6) versus MCI subjects (n = 11) (*p < .05).
Fig. 5
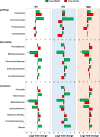
Differential effects of Mediterranean-style ketogenic diet (MMKD) and American Heart Association Diet (AHAD) on the functional analysis of the gut microbiome. Functional content of the gut microbiota was inferred by using PICRUSt based on the baseline and endpoint 16S amplicon sequencing data. Changes in the functional KEGG pathways during each dietary intervention were calculated by comparing the relative abundance of the KEGG orthologs and pathways (Level 2 and 3) at baseline vs. endpoint data and were interpreted in the form of mean Log2-fold change.
Fig. 6
Mediterranean-style ketogenic diet (MMKD) and American Heart Association Diet (AHAD) differently influence gut microbial metabolites in subjects clinically diagnosed with mild cognitive impairment (MCI) versus cognitively normal (CN) counterparts. Mean percent change in the fecal concentration of intestinal organic acids in all participants (n = 17) (a-d) and separately in CN (n = 6) versus MCI subjects (n = 11) (e-h) (*p < .05).
Fig. 7
Diet-induced changes in gut microbiome and fecal organic acids are associated with changes in cerebral spinal fluid (CSF) biomarkers of Alzheimer's disease (AD) in subjects clinically diagnosed with mild cognitive impairment (MCI) versus cognitively normal (CN) counterparts. Heat-map depicting the correlation patterns (Spearman R; *p < .05) of changes (percent-fold) in gut bacterial taxa and fecal organic acids with changes (percent-fold) in CSF biomarkers of AD in CN (n = 6) versus MCI subjects (n = 11) (e-h).
Similar articles
- Gut mycobiome and its interaction with diet, gut bacteria and alzheimer's disease markers in subjects with mild cognitive impairment: A pilot study.
Nagpal R, Neth BJ, Wang S, Mishra SP, Craft S, Yadav H. Nagpal R, et al. EBioMedicine. 2020 Sep;59:102950. doi: 10.1016/j.ebiom.2020.102950. Epub 2020 Aug 30. EBioMedicine. 2020. PMID: 32861197 Free PMC article. - Modified ketogenic diet is associated with improved cerebrospinal fluid biomarker profile, cerebral perfusion, and cerebral ketone body uptake in older adults at risk for Alzheimer's disease: a pilot study.
Neth BJ, Mintz A, Whitlow C, Jung Y, Solingapuram Sai K, Register TC, Kellar D, Lockhart SN, Hoscheidt S, Maldjian J, Heslegrave AJ, Blennow K, Cunnane SC, Castellano CA, Zetterberg H, Craft S. Neth BJ, et al. Neurobiol Aging. 2020 Feb;86:54-63. doi: 10.1016/j.neurobiolaging.2019.09.015. Epub 2019 Sep 26. Neurobiol Aging. 2020. PMID: 31757576 Free PMC article. - Effects of a ketogenic and low-fat diet on the human metabolome, microbiome, and foodome in adults at risk for Alzheimer's disease.
Dilmore AH, Martino C, Neth BJ, West KA, Zemlin J, Rahman G, Panitchpakdi M, Meehan MJ, Weldon KC, Blach C, Schimmel L, Kaddurah-Daouk R, Dorrestein PC, Knight R, Craft S; Alzheimer's Gut Microbiome Project Consortium. Dilmore AH, et al. Alzheimers Dement. 2023 Nov;19(11):4805-4816. doi: 10.1002/alz.13007. Epub 2023 Apr 5. Alzheimers Dement. 2023. PMID: 37017243 Free PMC article. - Diet-Microbiota-Brain Axis in Alzheimer's Disease.
Kincaid HJ, Nagpal R, Yadav H. Kincaid HJ, et al. Ann Nutr Metab. 2021;77 Suppl 2(Suppl 2):21-27. doi: 10.1159/000515700. Epub 2021 Apr 27. Ann Nutr Metab. 2021. PMID: 33906194 Free PMC article. Review. - The Microbiota-Gut-Brain Axis in Alzheimer's Disease: A Review of Taxonomic Alterations and Potential Avenues for Interventions.
Murray ER, Kemp M, Nguyen TT. Murray ER, et al. Arch Clin Neuropsychol. 2022 Feb 22;37(3):595-607. doi: 10.1093/arclin/acac008. Arch Clin Neuropsychol. 2022. PMID: 35202456 Free PMC article. Review.
Cited by
- To Keto or Not to Keto? A Systematic Review of Randomized Controlled Trials Assessing the Effects of Ketogenic Therapy on Alzheimer Disease.
Grammatikopoulou MG, Goulis DG, Gkiouras K, Theodoridis X, Gkouskou KK, Evangeliou A, Dardiotis E, Bogdanos DP. Grammatikopoulou MG, et al. Adv Nutr. 2020 Nov 16;11(6):1583-1602. doi: 10.1093/advances/nmaa073. Adv Nutr. 2020. PMID: 32597927 Free PMC article. - Diet and the Microbiota-Gut-Brain Axis: Sowing the Seeds of Good Mental Health.
Berding K, Vlckova K, Marx W, Schellekens H, Stanton C, Clarke G, Jacka F, Dinan TG, Cryan JF. Berding K, et al. Adv Nutr. 2021 Jul 30;12(4):1239-1285. doi: 10.1093/advances/nmaa181. Adv Nutr. 2021. PMID: 33693453 Free PMC article. Review. - The gut microbiota-brain axis in neurological disorders.
You M, Chen N, Yang Y, Cheng L, He H, Cai Y, Liu Y, Liu H, Hong G. You M, et al. MedComm (2020). 2024 Jul 20;5(8):e656. doi: 10.1002/mco2.656. eCollection 2024 Aug. MedComm (2020). 2024. PMID: 39036341 Free PMC article. Review. - The Gut Microbiome as a Component of the Gut-Brain Axis in Cognitive Health.
Gao W, Baumgartel KL, Alexander SA. Gao W, et al. Biol Res Nurs. 2020 Oct;22(4):485-494. doi: 10.1177/1099800420941923. Epub 2020 Jul 17. Biol Res Nurs. 2020. PMID: 32677447 Free PMC article. - Protective properties of Ophiopogonin D in DSS-induced colitis: insights into microbiota modulation.
Zhang T, Guo Z, Cheng X, Xia R, Lai S, Liao L. Zhang T, et al. Inflammopharmacology. 2024 Oct;32(5):3553-3570. doi: 10.1007/s10787-024-01531-x. Epub 2024 Jul 22. Inflammopharmacology. 2024. PMID: 39039348
References
- Association, A. s Alzheimer's disease facts and figures, an annual report. https://www.alz.org/alzheimers-dementia/facts-figures
- Hardy J., Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol Sci. 1991;12(10):383–388. - PubMed
- Le Page A., Dupuis G., Frost E.H., Larbi A., Pawelec G., Witkowski J.M. Role of the peripheral innate immune system in the development of Alzheimer's disease. Exp Gerontol. 2018;107:59–66. - PubMed
MeSH terms
Substances
Grants and funding
- R01 AG018915/AG/NIA NIH HHS/United States
- T32 OD010957/OD/NIH HHS/United States
- P30 AG049638/AG/NIA NIH HHS/United States
- UL1 TR001420/TR/NCATS NIH HHS/United States
- P30 AG021332/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous