Nitric oxide loading reduces sickle red cell adhesion and vaso-occlusion in vivo - PubMed (original) (raw)
Nitric oxide loading reduces sickle red cell adhesion and vaso-occlusion in vivo
Timothy J McMahon et al. Blood Adv. 2019.
Abstract
Sickle red blood cells (SSRBCs) are adherent to the endothelium, activate leukocyte adhesion, and are deficient in bioactive nitric oxide (NO) adducts such as S_-nitrosothiols (SNOs), with reduced ability to induce vasodilation in response to hypoxia. All these pathophysiologic characteristics promote vascular occlusion, the hallmark of sickle cell disease (SCD). Loading hypoxic SSRBCs in vitro with NO followed by reoxygenation significantly decreased epinephrine-activated SSRBC adhesion to the endothelium, the ability of activated SSRBCs to mediate leukocyte adhesion in vitro,_ and vessel obstruction in vivo. Because transfusion is frequently used in SCD, we also determined the effects of banked (SNO-depleted) red blood cells (RBCs) on vaso-occlusion in vivo. Fresh or 14-day-old normal RBCs (AARBCs) reduced epinephrine-activated SSRBC adhesion to the vascular endothelium and prevented vaso-occlusion. In contrast, AARBCs stored for 30 days failed to decrease activated SSRBC adhesivity or vaso-occlusion, unless these RBCs were loaded with NO. Furthermore, NO loading of SSRBCs increased _S_-nitrosohemoglobin and modulated epinephrine's effect by upregulating phosphorylation of membrane proteins, including pyruvate kinase, E3 ubiquitin ligase, and the cytoskeletal protein 4.1. Thus, abnormal SSRBC NO/SNO content both contributes to the vaso-occlusive pathophysiology of SCD, potentially by affecting at least protein phosphorylation, and is potentially amenable to correction by (S)NO repletion or by RBC transfusion.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
Graphical abstract
Figure 1.
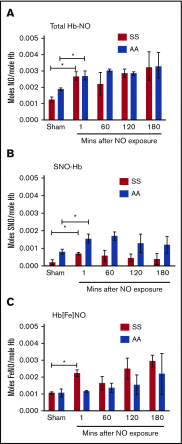
Exposure of SSRBCs to NO increases formation of stable Hb-bound NO. Total NO bound to Hb (A), SNO-Hb (B), and Hb[Fe]NO (C) was assayed by photolysis-chemiluminescence before (sham) and at varying times (in minutes) after RBC exposure to aqueous NO solution (1:250 NO:Hb ratio). Results are expressed as number of moles (S)NO per moles of Hb. Hb-bound NO in SSRBCS (SS) after exposure to NO is stable for at least 3 hours. The mean and standard error of the mean (SEM) of 3 independent experiments are shown for each set of conditions. *P < .05 for both normal RBCs (AA) and SSRBCs (SS) vs the respective sham. None of the subsequent changes from 1 to 180 minutes was statistically significant.
Figure 2.
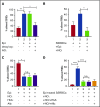
Exposure of SSRBCs to NO reduces both SSRBC adhesion and the ability of SSRBCs to activate mononuclear leukocyte adhesion. (A) NO loading of SSRBCs inhibits adhesion to ECs in vitro. SSRBCs were sham loaded (deoxygenation under helium/reoxygenation at room air) or NO loaded as described in "Materials and methods," followed by exposure to vehicle alone or to epinephrine (Epi) for 1 minute. Adhesion of SSRBCs to HUVECs was tested in intermittent flow condition assays. Results are presented as percent adherent SSRBCs at a shear stress of 1 dyne/cm2. Error bars show SEMs of 3 different experiments using blood samples from 3 different patient donors with SCD. **P < .002 for vehicle-treated vs Epi-treated; *P < .01 for sham-loaded Epi-treated vs NO-loaded Epi-treated. (B) NO prevents SSRBCs from inducing mononuclear leukocyte adhesion to ECs in vitro. SSRBCs were loaded with NO before epinephrine treatment. Adhesion of PBMCs pre-incubated with washed Epi-treated SSRBCs or washed NO-loaded Epi-treated SSRBCs were then assayed. Results are presented as percent adherent PBMCs at a shear stress of 1 dyne/cm2. Error bars show SEMs of 3 different experiments using blood samples from 3 different patient donors with SCD. *P = .0207 for PBMCs + Epi-treated SS vs PBMCs + NO-loaded Epi-treated SS. (C-D) The effect of SSRBC NO supplementation on adhesion in vitro is independent of release of NO itself. Adhesion of SSRBCs and PBMCs to HUVECs was tested in intermittent flow condition assays, and results are presented as percent adherent cells at a shear stress of 1 dyne/cm2. Error bars show SEMs of 3 different experiments using blood samples from 3 different patients with SCD for panels C and D, and 3 different healthy donors for panel D. (C) SSRBCs were treated with Epi for 1 minute, NO loaded followed by stimulation with Epi, NO loaded followed by stimulation with Epi and then incubation with free HbA, or NO loaded followed by stimulation with Epi then incubation with albumin (alb). *P = .0003 for Epi-treated vs NO-loaded Epi-treated. There was no statistically significant difference in adhesion of NO-loaded Epi-treated SSRBCs vs SSRBCs that had NO-loaded and Epi-treated and then incubated with either free HbA or alb. (D) SSRBCs were Epi-treated or NO loaded then Epi-treated, before coincubation with PBMCs in the presence of free HbA or alb. ****P < .0001 for PBMCs + Epi-treated SSRBCs vs PBMCs alone and PBMCs + NO-loaded Epi-treated SSRBCs vs PBMCs + Epi-treated SSRBCs. There was no statistically significant difference measured for adhesion of PBMCs + NO-loaded Epi-treated SSRBCs vs PBMCs + NO-loaded Epi-treated SSRBCs in the presence of free HbA or alb. n.s., not significant.
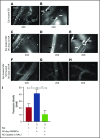
Figure 3.
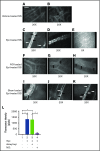
Replenishment of NO in SSRBCs reduces Epi-activated SSRBC adhesion to the endothelium and vessel occlusion in vivo. (A-K) Microscopic observations of postcapillary venules were conducted through implanted dorsal skin-fold window chambers after infusion of human SSRBCs into the tail vein of nude mice using 5× and 20× magnification. Vessels without adherent cells appear gray, due to the blurred fluorescence of rapidly moving SSRBCs. Infusion of vehicle-treated (n = 5; A-B), Epi-treated (n = 5; C-E), NO-loaded Epi-treated (n = 5; F-H), or sham-loaded Epi-treated (n = 5; I-K) human SSRBCs was performed. (A-E) Vehicle-treated human SSRBCs showed little adhesion to vessel walls (indicated by black arrows), whereas Epi-treated human SSRBCs showed marked adhesion to postcapillary venules, as indicated by black arrows, with intermittent vaso-occlusion, as indicated by white arrows. (F-K) In contrast, NO-loaded Epi-treated human SSRBCs displayed only minimal adhesion (indicated by black arrows) and no vaso-occlusion, whereas sham loading had no inhibitory effect on Epi-treated SSRBC adhesion (indicated by black arrows) and vaso-occlusion (indicated by white arrows). Scale bar, 50 μm. (L) Fluorescence intensity (pixels) represents fluorescence-labeled human SSRBC adhesion to vessel walls quantified by examining movies produced using 20× magnification. The values of segments of vessels analyzed were averaged among groups of animals to represent the mean fluorescence intensity. Error bars show SEM of 5 different experiments for each treatment. *P < .05 for either Epi-treated or sham-loaded Epi-treated vs vehicle-treated, and NO-loaded Epi-treated vs sham-loaded Epi-treated.
Figure 4.
Banked normal AARBCs stored for 30 days failed to reduce SSRBC adhesion to the vascular endothelium and vaso-occlusion in vivo. (A-M) Microscopic observations of postcapillary venules were conducted by using 10× and 20× magnification through implanted dorsal skin-fold window chambers after infusion of human SSRBCs into the tail vein of nude mice. Vessels without adherent cells appear gray, due to the blurred fluorescence of rapidly moving SSRBCs. Mice received infusion of vehicle-treated SSRBCs, Epi-treated SSRBCs, or Epi-treated SSRBCs mixed with fresh AARBCs or AARBCs stored for 14 or 30 days. Vehicle-treated SSRBCs adhered minimally to the endothelium in vivo (A-B), whereas Epi dramatically increased SSRBC adhesion (indicated by black arrows) and vaso-occlusion (indicated by white arrows) (C-D). Infusion of either fresh (“0 day”) AARBCs mixed with epi-treated SSRBCs (E-G), or AARBCs stored for 14 days mixed with Epi-treated SSRBCs (H-J), dramatically reduced SSRBC adhesion and vascular stasis compared with Epi-stimulated SSRBCs alone. In contrast, admixture with AARBCs stored for 30 days (K-M) failed to decrease adherence of Epi-activated SSRBCs to the endothelium (indicated by black arrows) and vascular occlusion (indicated by white arrows). (N) Fluorescence intensity (pixels) representative of human SSRBC adhesion to vessels. Movies (20× magnification) were used to quantify fluorescence intensity induced by adherent human SSRBCs in animals infused with fluorescence-labeled SSRBCs treated as described in panels A-M (n = 5 for each treatment). The values were averaged among groups of animals to represent the mean fluorescence intensity. Error bars show SEM. **P < .05 for Epi-treated SS vs vehicle-treated SS, and Epi-treated SS + fresh AA vs Epi-treated SS. ***P < .05 for Epi-treated SS + 14-day-old AARBCs vs Epi-treated SS.
Figure 5.
Loading banked (30 days old) AARBCs with NO decreased SSRBC adhesion and vaso-occlusion in vivo. (A-H) Inhibition of SSRBC adhesion with NO-loaded 30-day-old AARBCs was performed as described in "Materials and methods." Epi-treated SSRBCs (n = 5) exhibited marked adhesion to postcapillary venules, as indicated by black arrows, with intermittent vaso-occlusion as indicated by white arrows. Coinfusion with banked (30-day-old) AARBCs (n = 5) had no effect on Epi-treated SSRBC adhesion to postcapillary venules (indicated by black arrows) and vaso-occlusion (indicated by white arrows), whereas coinfusion with NO-loaded banked (30-day-old) AARBCs (n = 5) markedly inhibited adhesion of Epi-treated SSRBCs to postcapillary vessels (black arrows) with no vaso-occlusion. (I) Venule segments were analyzed by fluorescence intensity to quantify SSRBC adhesion. The values were averaged among groups of animals (n = 5 for each treatment) to represent the mean fluorescence intensity. Error bars show SEM of 5 different experiments. *P = .0207 for Epi-treated SS + old AA compared with Epi-treated SS; **P = .0046 for Epi-treated SS + old AA vs Epi-treated SS + NO-loaded old AA.
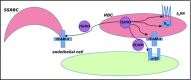
Figure 6.
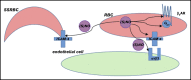
Model of NO-induced inhibition of SSRBC adhesion. RBC NO or SNO may modulate (eg, inhibit) intercellular adhesion via autocrine mechanisms such as by binding to reactive cysteine residues in the relevant G protein–coupled receptors such as the beta2-adrenergic receptor (β2AR), its downstream signaling partners such as the S-type G-protein (Gαs), or an adhesion receptor such as LW/ICAM-4. Alternatively, SNO (but not NO) exported from RBCs may inhibit adhesion by acting on adjacent cells in paracrine fashion; for example, during RBC-endothelial cell (or RBC-leukocyte) contact, possibly functionally modifying a counterreceptor such as α-v-β-3 (αvβ3) integrin, or when transfused AARBCs mix with native, activated SSRBCs.
Similar articles
- Disrupting the vicious cycle created by NOX activation in sickle erythrocytes exposed to hypoxia/reoxygenation prevents adhesion and vasoocclusion.
MacKinney A, Woska E, Spasojevic I, Batinic-Haberle I, Zennadi R. MacKinney A, et al. Redox Biol. 2019 Jul;25:101097. doi: 10.1016/j.redox.2019.101097. Epub 2019 Jan 11. Redox Biol. 2019. PMID: 30661992 Free PMC article. - MEK inhibitors, novel anti-adhesive molecules, reduce sickle red blood cell adhesion in vitro and in vivo, and vasoocclusion in vivo.
Zennadi R. Zennadi R. PLoS One. 2014 Oct 20;9(10):e110306. doi: 10.1371/journal.pone.0110306. eCollection 2014. PLoS One. 2014. PMID: 25330306 Free PMC article. - Gαs proteins activate p72(Syk) and p60-c-Src tyrosine kinases to mediate sickle red blood cell adhesion to endothelium via LW-αvβ3 and CD44-CD44 interactions.
Chiou E, Zennadi R. Chiou E, et al. Int J Biochem Cell Biol. 2015 Aug;65:40-51. doi: 10.1016/j.biocel.2015.05.013. Epub 2015 May 23. Int J Biochem Cell Biol. 2015. PMID: 26007235 - Sickle cell vaso-occlusion.
Chiang EY, Frenette PS. Chiang EY, et al. Hematol Oncol Clin North Am. 2005 Oct;19(5):771-84, v. doi: 10.1016/j.hoc.2005.08.002. Hematol Oncol Clin North Am. 2005. PMID: 16214643 Review. - Erythrocyte adhesion in sickle cell disease.
Parise LV, Telen MJ. Parise LV, et al. Curr Hematol Rep. 2003 Mar;2(2):102-8. Curr Hematol Rep. 2003. PMID: 12901140 Review.
Cited by
- Red Blood Cell Deformability, Vasoactive Mediators, and Adhesion.
McMahon TJ. McMahon TJ. Front Physiol. 2019 Nov 15;10:1417. doi: 10.3389/fphys.2019.01417. eCollection 2019. Front Physiol. 2019. PMID: 31803068 Free PMC article. Review. - Endothelial LAT1 (SLC7A5) Mediates S-Nitrosothiol Import and Modulates Respiratory Sequelae of Red Blood Cell Transfusion In Vivo.
Zhu H, Auten RL, Whorton AR, Mason SN, Bock CB, Kucera GT, Kelleher ZT, Vose AT, McMahon TJ. Zhu H, et al. Thromb Haemost. 2024 Jul;124(7):656-668. doi: 10.1055/s-0044-1782182. Epub 2024 Mar 22. Thromb Haemost. 2024. PMID: 38519039 Free PMC article. - Modulation of the allosteric and vasoregulatory arms of erythrocytic oxygen transport.
Wise TJ, Ott ME, Joseph MS, Welsby IJ, Darrow CC, McMahon TJ. Wise TJ, et al. Front Physiol. 2024 Jun 10;15:1394650. doi: 10.3389/fphys.2024.1394650. eCollection 2024. Front Physiol. 2024. PMID: 38915775 Free PMC article. Review. - The enzymatic function of the honorary enzyme: S-nitrosylation of hemoglobin in physiology and medicine.
Premont RT, Singel DJ, Stamler JS. Premont RT, et al. Mol Aspects Med. 2022 Apr;84:101056. doi: 10.1016/j.mam.2021.101056. Epub 2021 Nov 28. Mol Aspects Med. 2022. PMID: 34852941 Free PMC article. Review. - Red Blood Cell-Mediated S-Nitrosohemoglobin-Dependent Vasodilation: Lessons Learned from a β-Globin Cys93 Knock-In Mouse.
Premont RT, Reynolds JD, Zhang R, Stamler JS. Premont RT, et al. Antioxid Redox Signal. 2021 Apr 20;34(12):936-961. doi: 10.1089/ars.2020.8153. Epub 2020 Jul 23. Antioxid Redox Signal. 2021. PMID: 32597195 Free PMC article. Review.
References
- Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. N Engl J Med. 2008;359(21):2254-2265. - PubMed
- Aslan M, Ryan TM, Townes TM, et al. . Nitric oxide-dependent generation of reactive species in sickle cell disease. Actin tyrosine induces defective cytoskeletal polymerization. J Biol Chem. 2003;278(6):4194-4204. - PubMed
- French JA II, Kenny D, Scott JP, et al. . Mechanisms of stroke in sickle cell disease: sickle erythrocytes decrease cerebral blood flow in rats after nitric oxide synthase inhibition. Blood. 1997;89(12):4591-4599. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL079915/HL/NHLBI NIH HHS/United States
- R01 GM113838/GM/NIGMS NIH HHS/United States
- I01 BX003478/BX/BLRD VA/United States
- R01 HL137930/HL/NHLBI NIH HHS/United States
- R01 HL107608/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical